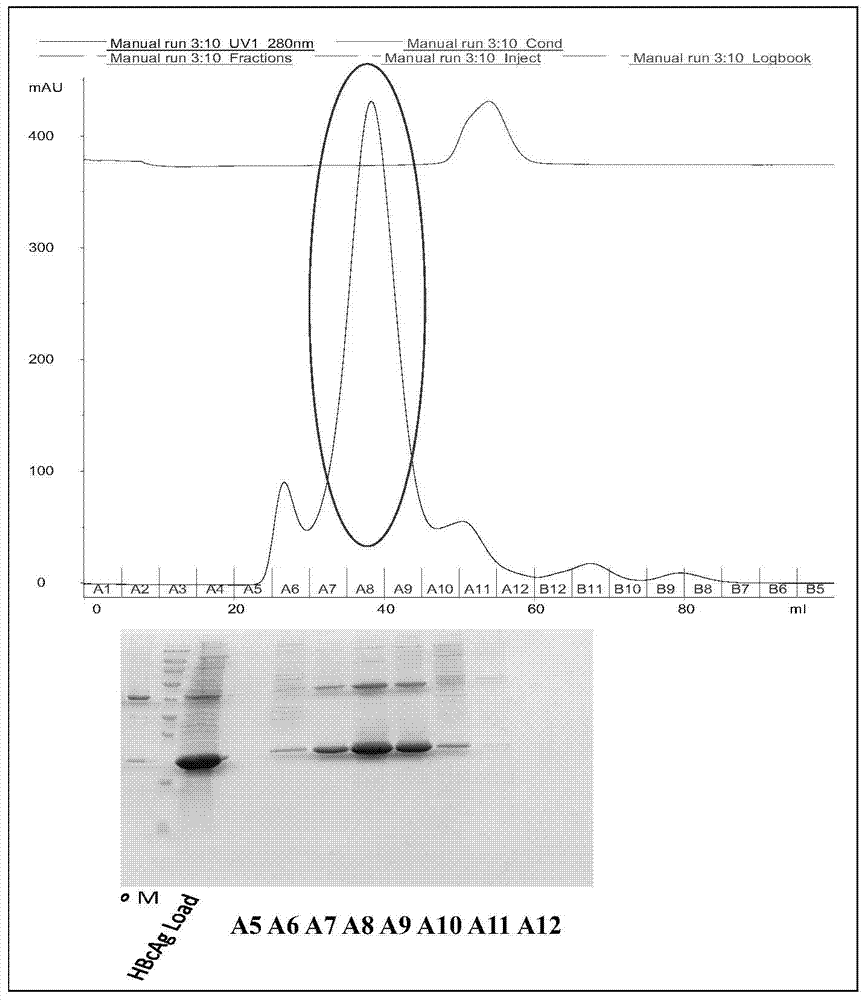
A method for purifying prokaryotic cells expressing virus-like particles
A virus-like particle and purification method technology, applied in the field of virus-like particle purification, can solve the problems of HBcAg purity and the difficulty of meeting quality standards for residual substances, and achieve the effects of easy amplification, short reaction time, high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Analysis and synthesis of HBcAg gene
[0050] There are multiple serotypes and genotypes of hepatitis B virus. In the present embodiment, a total of 9125 strains of HBcAg target gene sequences of various genotypes were obtained from NCBI, wherein the genotype classification is as shown in Table 1 below, using Mega software (Mega5 .0, BorlandDelphi) to analyze the retrieved HBcAg of each genotype, so as to obtain the consensus sequence of each genotype (Consensus sequence), a total of 30 subtypes of HBcAg consensus gene sequence (as shown in Table 1), take the conservativeness greater than 50 % of amino acid categories, the mutation rate of the full-length HBcAg sequences of each genotype was analyzed one by one, and then the consensus sequence analysis (Consensus analysis) was performed again to obtain the final HBcAg full-length consensus sequence of 183 amino acids, which was used as The source of the HBcAg gene in the present invention, the consensus seque...
Embodiment 2
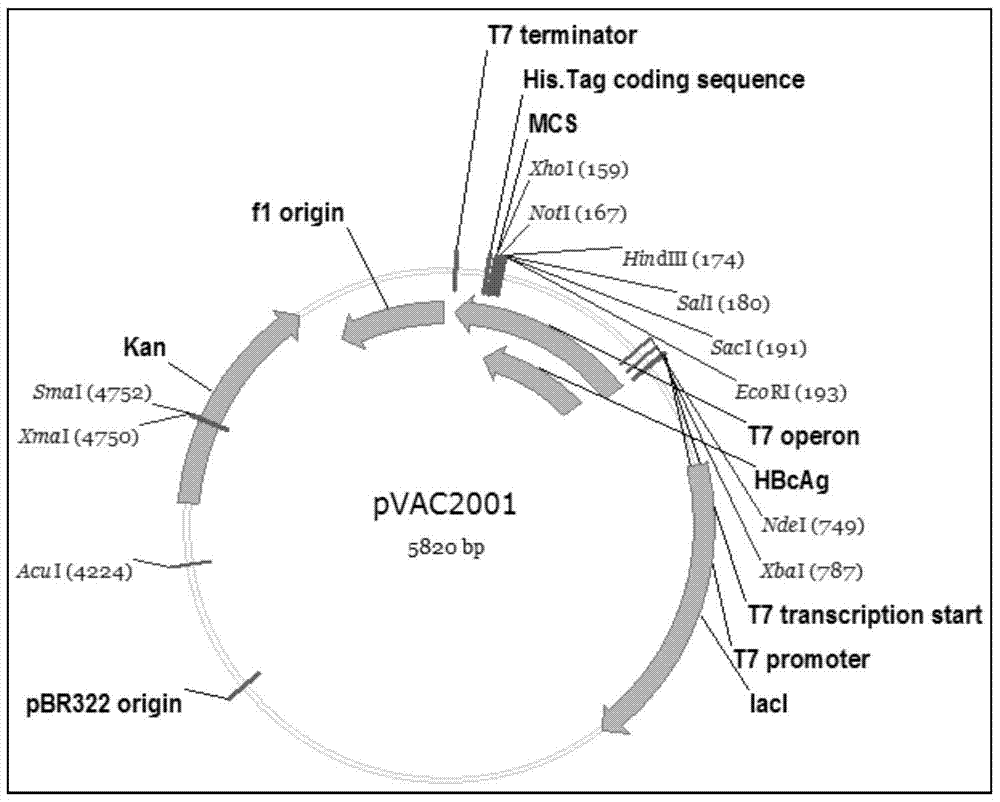
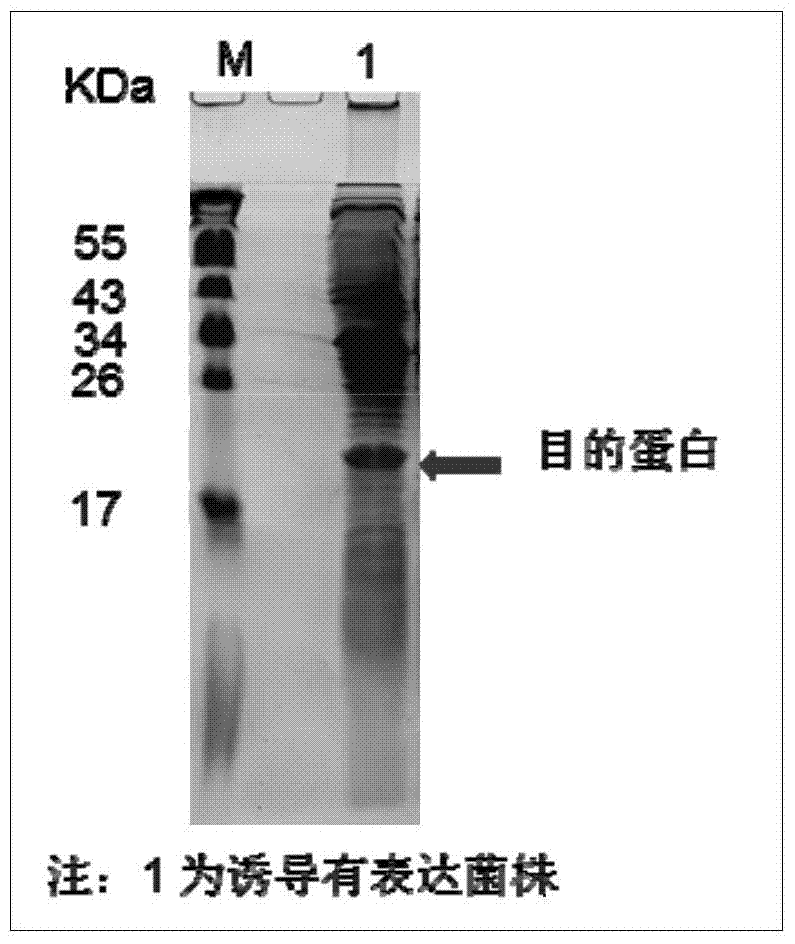
[0056] Example 2. HBcAg expression vector construction and induction identification
[0057] Using the synthesized Con183 nucleotide fragment as a template, Con183F (SEQ.ID No:3) as a forward primer (NdeI restriction site was introduced at the 5' end), and Con183R (SEQ.ID No:4) as a reverse primer (The 3' end introduces an EcoRI restriction site). The PCR reaction was carried out in a PCR instrument (ABI) according to the conditions shown in Table 2.
[0058] SEQ.ID No:3: Forward primer (NdeI): 5'-GGAATTCCATATGGACATTGACCCG-3'
[0059] SEQ.ID No:4: Reverse primer (EcoRI): 5'-CCGGAATTCCTAACACTGGCTTTC-3'
[0060] Table 2PCR reaction conditions
[0061]
[0062] A fragment of about 560bp was obtained after PCR amplification. The amplified product obtained above was connected to a commercially available pMD18-T vector (manufactured by TAKARA Company), and named pMD18-T-Con183, identified by NdeI / EcoRI digestion and sequenced to obtain the enzyme cleavage site added at both e...
Embodiment 3
[0064] Example 3. Fermentation of HBcAg
[0065] Melt the bacteria glycerin tube suspension stored in the -80°C refrigerator at room temperature and shake well, then streak on the LB plate, culture at 37°C for 8-12 hours to grow a single colony and microscopically check for no bacteria, and the bacteria can be used for liquid after rejuvenation. nourish. Select a single colony on the rejuvenation plate and inoculate it into a 500mL Erlenmeyer flask filled with 50mL LB liquid medium, culture at 37°C, 200rpm for 10h, and the OD600 reaches about 3.0 as the first-class seed.
[0066] The primary seeds were inoculated into a 2L Erlenmeyer flask with 300 mL of LB liquid medium at 5% inoculum volume, and cultured at 37°C and 200 rpm for 14 hours until the OD600 reached about 4.0 as the secondary seeds.
[0067] After microscopic examination of the second-level seeds confirms that there is no miscellaneous bacteria, 5% inoculum volume (250ml) is aseptically inoculated into a fermente...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com