Improved method for extracting alkali metal compound from solid fluorine reconstruction lepidolite
A technology of alkali metal compounds and lepidolite, applied in the directions of alkali metal compounds, chemical instruments and methods, rubidium/cesium/francium compounds, etc. The effect of eliminating uneven mixing, reducing production costs and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
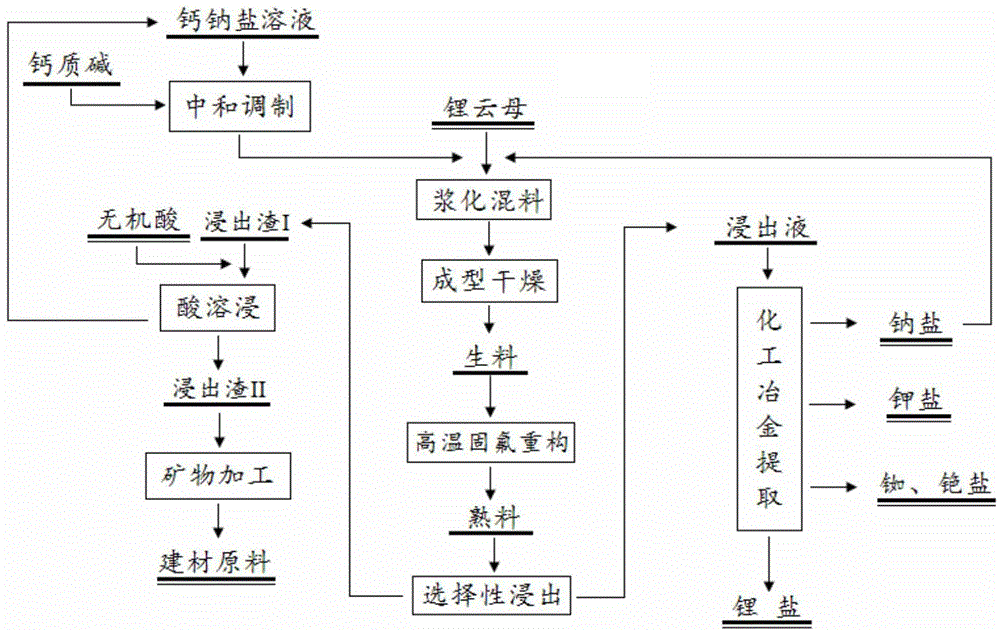
[0054] Mix lepidolite powder and calcium sodium chloride solution according to the ratio of lepidolite: calcium sodium chloride: water = 1000:820:2000, stir and slurry for 20 minutes; dry and granulate the uniformly mixed slurry to make particle size 25 ~ 30mm, and then dried to less than 5% of raw material moisture. The granular raw meal is heat-treated in a heating furnace at 720°C for 20 minutes to produce clinker; the high-temperature flue gas is cooled and absorbed by water to make acid. The clinker is soaked in water according to the initial liquid-solid mass ratio of 2:1, filtered and separated, and the slag is washed; after the solution is purified and concentrated, it is transferred to lithium carbonate for precipitation; after the lithium precipitation mother liquor is treated, it is transferred to rubidium and cesium salt for extraction.
[0055] The main mineral phase composition of the leaching slag dry basis is: CaF 2 7%; CaO.Al 2 o 3 .2SiO2 2 63%; CaO.SiO 2...
Embodiment 2
[0059] Mix lepidolite powder and calcium sodium chloride solution according to the ratio of lepidolite: calcium sodium chloride: water = 1000:710:1500, stir and slurry for 30 minutes; dry and granulate the uniformly mixed slurry to make particle size 5 ~ 10mm, and then dried to less than 5% of the raw material moisture. The granular raw meal is heat-treated in a heating furnace at 880°C for 40 minutes to produce clinker; the high-temperature flue gas is cooled and absorbed by water to make acid. The clinker is soaked in water according to the initial liquid-solid mass ratio of 2:1, filtered and separated, and the slag is washed; after the solution is purified and concentrated, it is transferred to lithium carbonate for precipitation; after the lithium precipitation mother liquor is treated, it is transferred to rubidium and cesium salt for extraction.
[0060] The main mineral phase composition of the leaching slag dry basis is: CaF 2 6.7%; CaO.Al 2 o 3 .2SiO2 2 61%; CaO.S...
Embodiment 3
[0064] Mix lepidolite powder and calcium sodium chloride solution according to the ratio of lepidolite: calcium sodium chloride: water = 1000:760:1800, stir and slurry for 25 minutes; dry and granulate the uniformly mixed slurry to make particle size 15-20mm, and then dried to less than 5% of the raw material moisture. The granular raw meal is heat-treated in a heating furnace at 800°C for 30 minutes to produce clinker; the high-temperature flue gas is cooled and absorbed by water to make acid. The clinker is soaked in water according to the initial liquid-solid mass ratio of 2:1, filtered and separated, and the slag is washed; after the solution is purified and concentrated, it is transferred to lithium carbonate for precipitation; after the lithium precipitation mother liquor is treated, it is transferred to rubidium and cesium salt for extraction.
[0065] The main mineral phase composition of the leaching slag dry basis is: CaF 2 6.9%; CaO.Al 2 o 3 .2SiO2 2 61.7%; CaO....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com