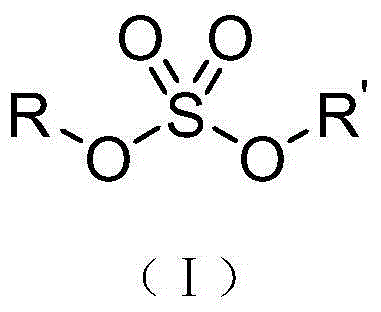
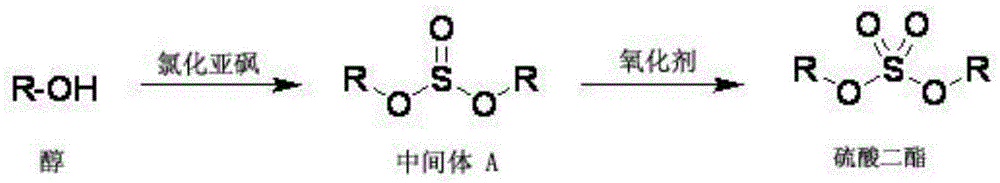
Method for compounding high-purity sulfuric acid esters
A technology of a sulfuric acid diester and a synthesis method, applied in the field of organic chemistry, can solve the problems of inability to truly meet the requirements of large-scale industrial production, large material loss, production failure, etc., and achieve easy industrial-scale production, low pollution, and simple production operations. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of diisobutyl sulfate
[0031] (1) Preparation of diisobutyl sulfite
[0032] In a dry 500 ml three-necked flask, add anhydrous isobutanol (200 g, 2.7 mol), cool to 0-10° C. with ice water, add thionyl chloride (107 g, 0.9 mol) dropwise under rapid stirring, After the dropwise addition was completed, the temperature was naturally raised to room temperature, and stirring was continued for 2 hours. Spot the plate to track the reaction. When it shows a single point, the reaction is over. The reaction time is 2 hours. Add 100 ml of saturated sodium bicarbonate aqueous solution to the reaction system, stir for 10 minutes, let stand to separate the layers, and wash the organic layer twice with water. (50ml×2) and dried to obtain a colorless oil (187g), yield 99%.
[0033] (2) Preparation of diisobutyl sulfate (potassium permanganate oxidation method)
[0034] The diisobutyl sulfite (187 grams) obtained above was dissolved in a mixed solvent of 3...
Embodiment 2
[0035] Embodiment 2: the synthesis of diisobutyl sulfate
[0036] (1) Preparation of diisobutyl sulfite
[0037] In a dry 500 ml three-necked flask, add anhydrous isobutanol (185 g, 2.5 mol), cool to 0-10 °C with ice water, add thionyl chloride (118 g, 1 mol) dropwise under rapid stirring, drop After the addition was completed, the temperature was naturally raised to room temperature, and stirring was continued for 2 hours. The dot plate tracks the reaction, and when it appears as a single dot, the reaction is complete. The reaction time was 20h, and 100 ml of saturated aqueous sodium bicarbonate solution was added to the reaction system, and after stirring for 10 minutes, the layers were left to stand, and the organic layer was washed twice with water (50 ml × 2), and dried to obtain a colorless oil ( 184 grams), yield 95%.
[0038] (2) Preparation of diisobutyl sulfate (hydrogen peroxide oxidation method)
[0039] The diisobutyl sulfite (184 grams) obtained above was dis...
Embodiment 3
[0040] Embodiment 3: the synthesis of diisobutyl sulfate
[0041] (1) Preparation of diisobutyl sulfite
[0042] In a dry 500 ml three-necked flask, add anhydrous isobutanol (163 g, 2.2 mol), cool to 0-10 ° C with ice water, add thionyl chloride (118 g, 1 mol) dropwise under rapid stirring, drop After the addition was completed, the temperature was naturally raised to room temperature, and stirring was continued for 2 hours. The dot plate tracks the reaction, and when it appears as a single dot, the reaction is complete. Reaction time is 10, in reaction system, add 100 milliliters of saturated sodium bicarbonate aqueous solutions, after stirring for 10 minutes, leave standstill layering, organic layer washes with water twice again (50 milliliters * 2), dry, obtain colorless oil ( 182 grams), yield 94%.
[0043] (2) Preparation of diisobutyl sulfate (oxone oxidation method)
[0044]The diisobutyl sulfite obtained above (182 g) was dissolved in 400 ml of tetrahydrofuran / wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com