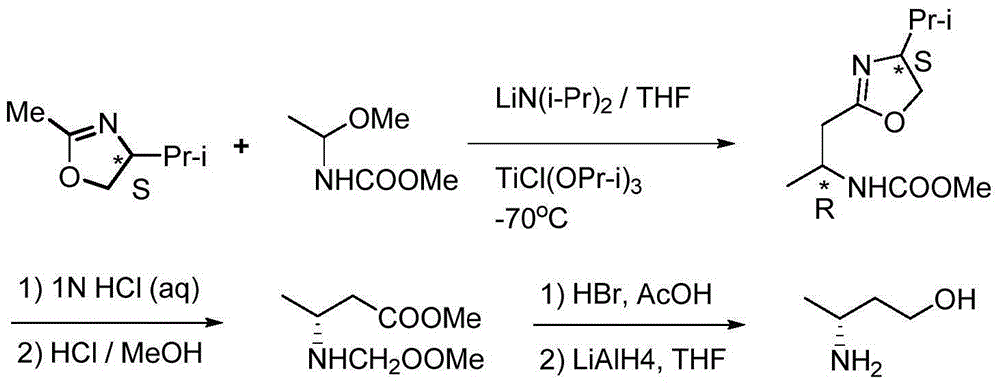
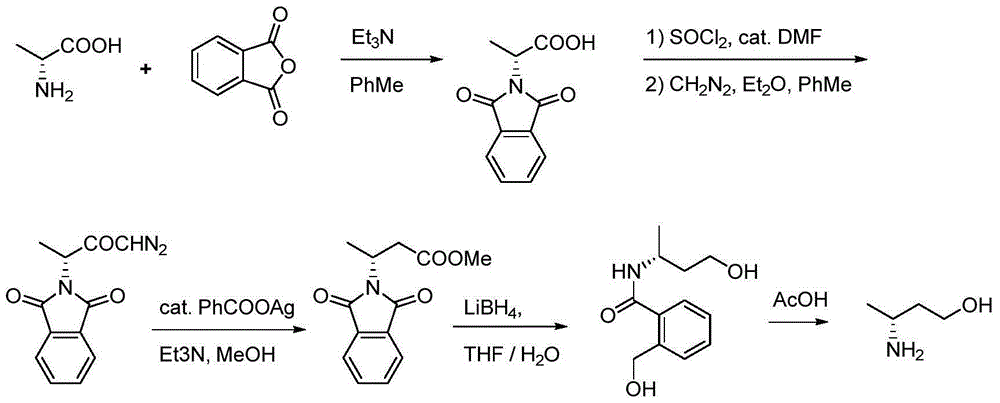
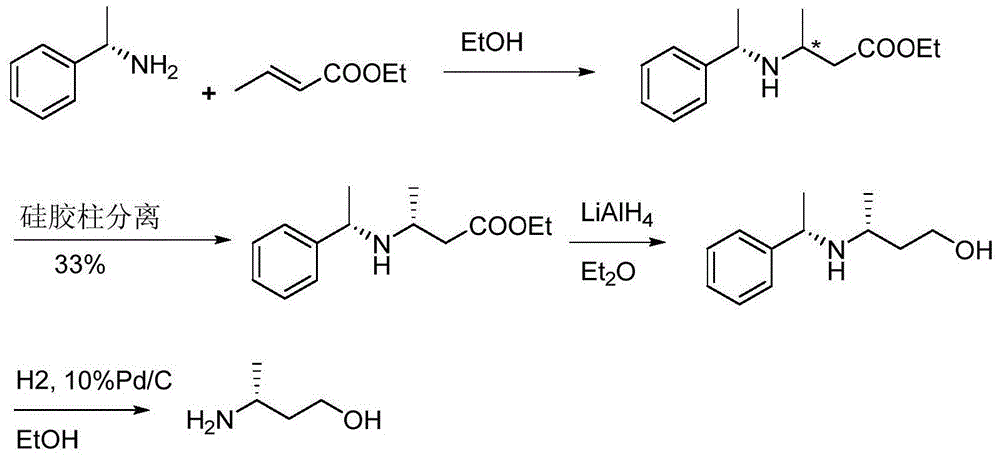
Method for preparing 3(R)/(S)-amidogen-1-butanol
A technology of -butanol and -amino, which is applied in the field of preparation of chiral drugs, can solve the problems of low yield, low yield of carboxyl group reduction reaction, high price of chiral phenylethylamine, etc., and achieve high yield and condensation reaction The effect of low yield and difficult waste liquid treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 3-benzamido-2-butenoic acid ethyl ester (I)
[0060] Add 130g of ethyl acetoacetate, 127g of benzamide, 17.2g of p-toluenesulfonic acid and 400mL of cyclohexane into a three-necked reaction flask equipped with a thermometer, stirring and water separator in turn, heat, reflux, and add cyclohexane Bring water, after about 18g of water is separated, the reaction is over. Evaporate cyclohexane under reduced pressure (recoverable for mechanical use), cool to room temperature, add 150mL methyl tert-butyl ether, filter after stirring, then rinse the filter cake once with 20mL methyl tert-butyl ether, dry the filter cake Recover and apply mechanically (containing p-toluenesulfonic acid and a small amount of unreacted benzamide), combine the filtrate and washing liquid, wash twice with 10% aqueous sodium carbonate solution, then wash twice with water, evaporate the solvent methyl tert-butyl ether ( can be recycled and applied), add 50mL cyclohexa...
Embodiment 2
[0061] The preparation of embodiment 2 3-benzamido-2-butenoic acid methyl ester (I)
[0062] With the operating procedure and aftertreatment method of embodiment 1, by 128g methyl acetoacetate, 127g benzamide, 17.2g p-toluenesulfonic acid and 400mL cyclohexane, make 3-benzamido-2-butenoic acid Methyl ester (I), white solid, 199g, yield 91%, content 99.0% (HPLC method).
Embodiment 3
[0063] Example 3 Preparation of 3(R)-benzamido ethyl butyrate (II)
[0064] Take 150g 3‐benzamido‐2‐butenoic acid ethyl ester, 750mL methanol, put into a clean pressure reactor, under nitrogen protection, then add 60mg chiral rhodium‐bisphosphine ligand catalyst R‐xyl‐ BINAP-cymene-Rucl 2 . After sequentially replacing with nitrogen and hydrogen, under the conditions of hydrogen pressure 1.0MPa and temperature 70-80°C, catalytic hydrogenation for 24 hours, after the reaction is completed, cool to room temperature, replace with nitrogen, take out the reaction liquid, filter, and recover the filtrate by vacuum distillation Solvent methanol (can be used mechanically in the same procedure of this step in the next batch of reactions), add 1000mL methyl tert-butyl ether to the residue to make a slurry, filter (after the filtrate is distilled, the recovered methyl tert-butyl ether can be used in the next batch of reactions. Apply mechanically in the same procedure of step), filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com