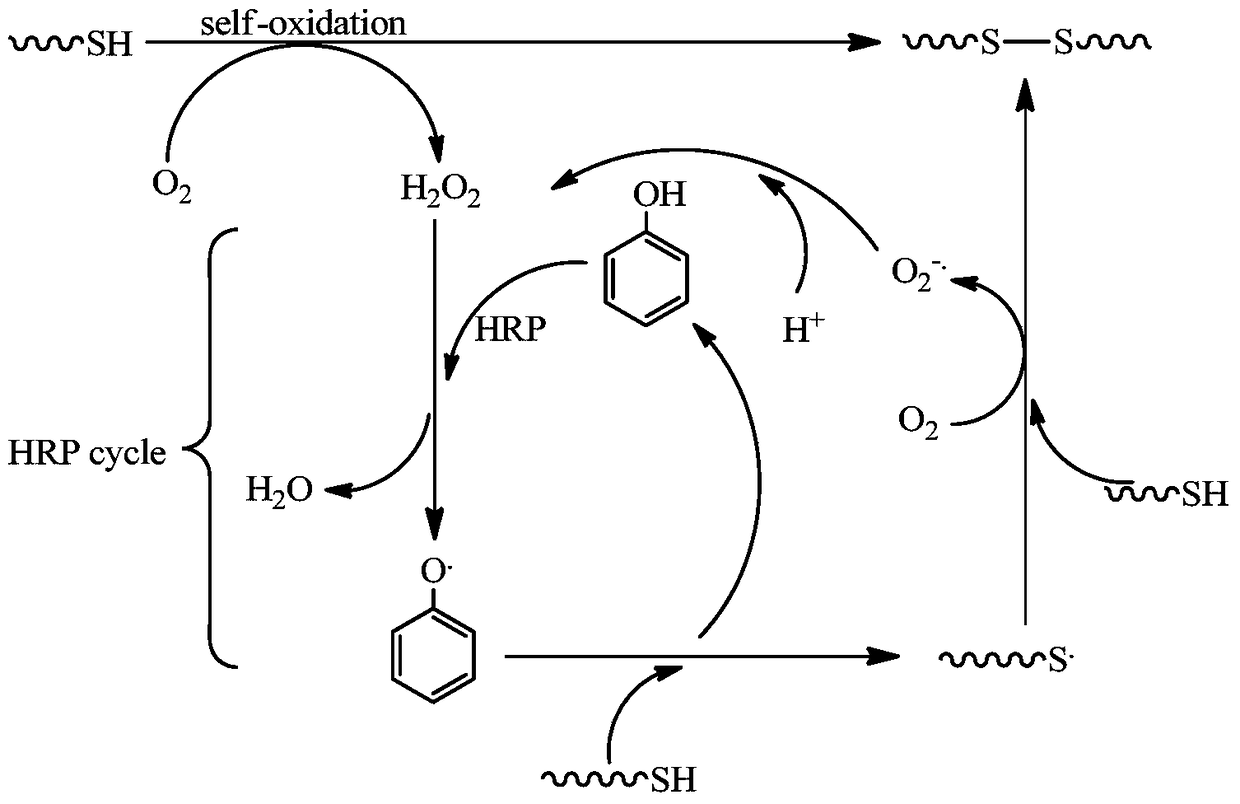
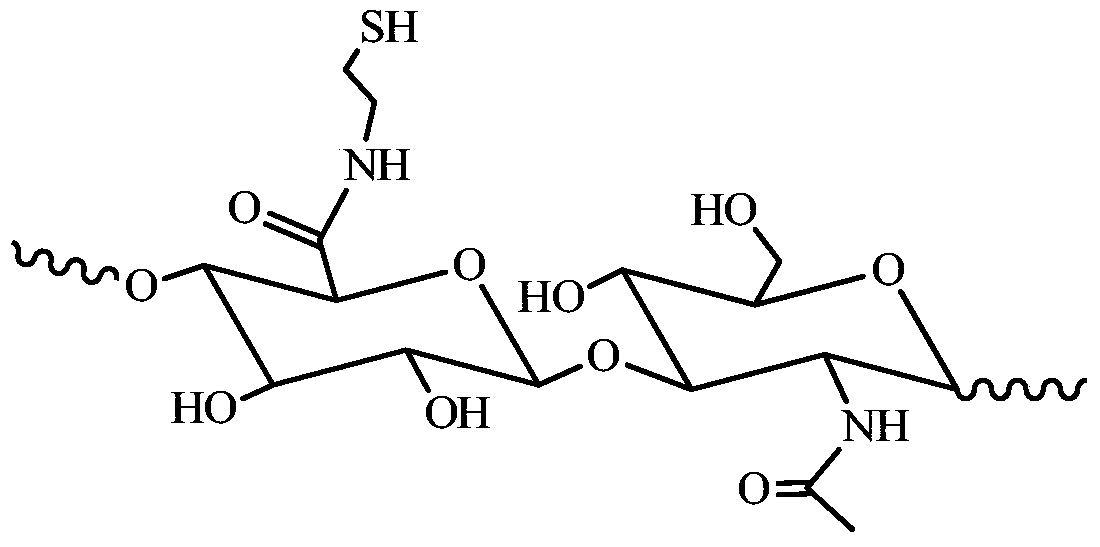
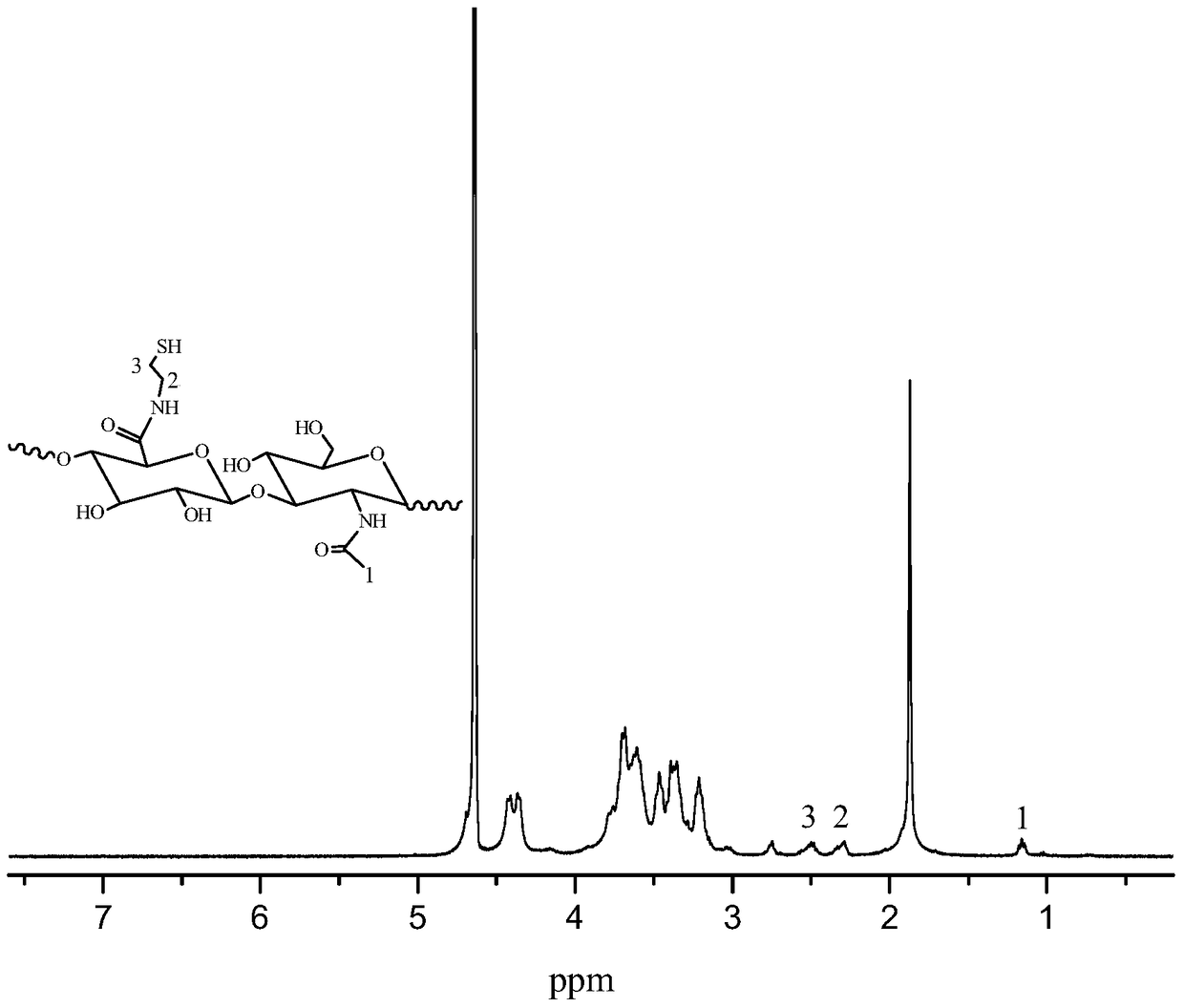
A kind of natural polymer hydrogel of enzyme-catalyzed disulfide bond cross-linking and its preparation method
An enzyme-catalyzed disulfide bond, natural polymer technology, applied in the field of medical biomaterials, can solve the problems of reduced enzyme activity, poor hydrogel uniformity, affecting the activity of loaded drugs, etc., achieving fast cross-linking speed, simple operation, excellent Effects of Biocompatibility and Biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of thiol-grafted hyaluronic acid macromolecule (HA-SH).
[0040] Sodium hyaluronate (2 g, 5 mmol) was weighed and dissolved in 100 mL of double distilled water, and stirred at room temperature with a magnetic stirrer until completely dissolved. Weigh N-hydroxysuccinimide (NHS) (2.3g, 20mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCL) (3.832g , 20 mmol) was added to the above-mentioned completely dissolved sodium hyaluronate solution, the pH value of the solution was adjusted to about 5.4, and activated at room temperature for 1 h. Cystamine dihydrochloride (3.372g, 15mmol) was weighed and added to the above activated solution, and stirred at room temperature for 48h. The reaction solution was transferred into a dialysis bag (MWCO3500) and dialyzed with twice distilled water for 24 hours. Weigh D,L-dithiothreitol (DTT) (4.6275g, 30mmol) and add it to the above reaction solution that has been dialyzed for 24h, adjust the pH value ...
Embodiment 2
[0042] Preparation of chitosan macromolecule grafted with mercapto groups (CS-SH)
[0043] Weigh chitosan (0.5 g) and dissolve it in 50 mL of double distilled water, add 2 mL of 1 mol / L hydrochloric acid, stir at room temperature with a magnetic stirrer until completely dissolved, and adjust the pH to 6 with 1 mol / L NaOH. Weigh N-acetylcysteine-L-amino acid (NAC) (2.3563g, 14.4mmol), N-hydroxysuccinimide (NHS) (2.0g, 17.3mmol), 1-(3-dimethylaminopropyl Base)-3-ethylcarbodiimide hydrochloride (EDC.HCL) (3.5 g, 18.3 mmol) was dissolved in 6 mL of N,N-dimethylformamide (DMF). The above DMF mixed solution was added dropwise to the completely dissolved chitosan solution, the pH value of the solution was kept at about 6.0, and the reaction was stirred at room temperature for 24 hours. The reaction solution was transferred into a dialysis bag (MWCO3500) and dialyzed with twice distilled water for 24 hours. Weigh D,L-dithiothreitol (DTT) (2.31g, 15mmol) and add it to the above react...
Embodiment 3
[0045] Preparation of polyglutamic acid macromolecules grafted with thiol groups (PGA-SH)
[0046] Sodium polyglutamate (0.3 g) was weighed and dissolved in 30 mL of double distilled water, and stirred at room temperature with a magnetic stirrer until completely dissolved. Weigh N-hydroxysuccinimide (NHS) (0.2624g, 2.28mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCL) (1.14 g, 5.94 mmol) was added to the above-mentioned completely dissolved sodium polyglutamate solution, the pH value of the solution was adjusted to about 6.0, and activated at room temperature for 1 h. Cysteamine hydrochloride (0.675g, 5.94mmol) was weighed and added to the above activated solution, and stirred at room temperature for 24h. The reaction solution was transferred into a dialysis bag and dialyzed with 1 mM hydrochloric acid for 1 day, 1 wt% NaCl solution for 1 day, and 1 mM hydrochloric acid for 1 day. After the dialysis, the reaction solution was quickly frozen with li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com