Good-compatibility and absorbable medical suture line and production method thereof
A suture and compatibility technology, applied in the field of medical sutures, can solve the problems of slow degradation rate, easy cell adhesion and supply, etc., to achieve the effect of increasing smoothness, reducing tissue drag, and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
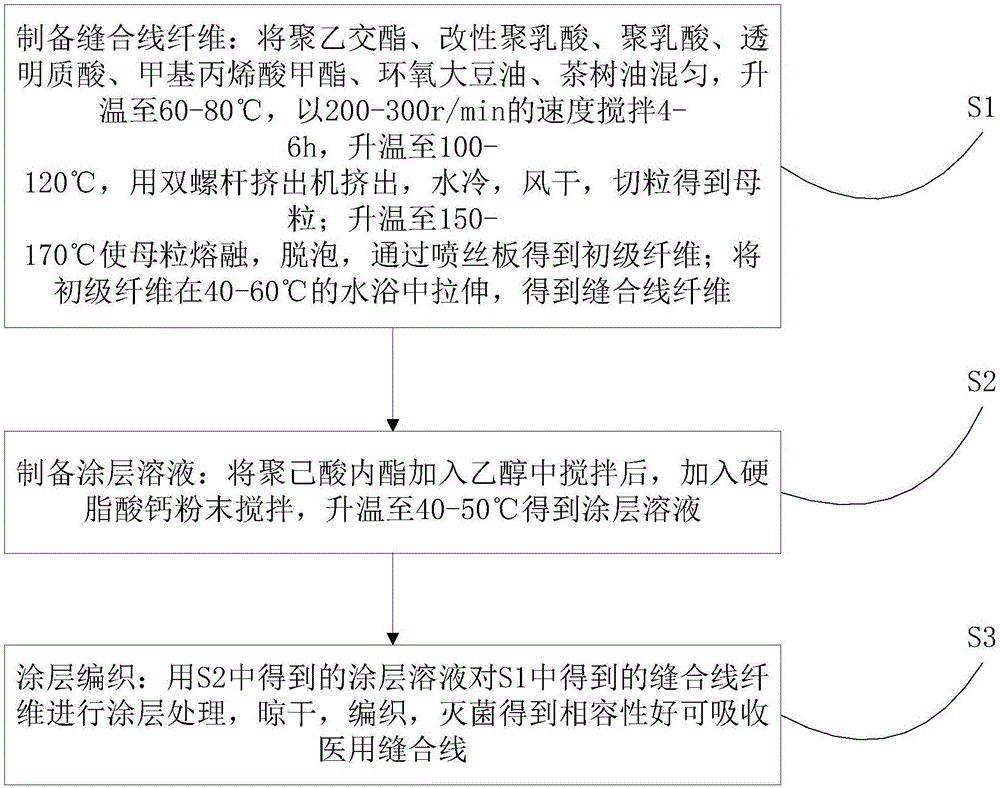
[0031] Reference figure 1 , The method for preparing an absorbable medical suture with good compatibility provided by the present invention includes the following steps:
[0032] S1. Preparation of suture fiber: mix polyglycolide, modified polylactic acid, polylactic acid, hyaluronic acid, methyl methacrylate, epoxidized soybean oil, and tea tree oil. -300r / min stirring for 4-6h, heating to 100-120℃, extruding with twin screw extruder, water cooling, air drying, pelletizing to obtain masterbatch; heating to 150-170℃ to melt masterbatch and defoam , Obtain primary fibers through a spinneret; stretch the primary fibers in a water bath at 40-60°C to obtain suture fibers;
[0033] S2. Preparation of coating solution: after adding polycaprolactone to ethanol and stirring, adding calcium stearate powder and stirring, and heating to 40-50°C to obtain a coating solution;
[0034] S3. Coating and knitting: coating the suture fiber obtained in S1 with the coating solution obtained in S2, dry...
Embodiment 1
[0037] A compatible and absorbable medical suture thread. Its raw materials by weight include: 40 parts of polyglycolide, 10 parts of modified polylactic acid, 70 parts of polylactic acid, 10 parts of hyaluronic acid, and 7 parts of methyl methacrylate. Parts, 4 parts epoxidized soybean oil, 4 parts tea tree oil, 1 part polycaprolactone, 0.3 parts calcium stearate, 15 parts ethanol;
[0038] Among them, the preparation method of modified polylactic acid is as follows: add 2 parts of polylactic acid to 20 parts of chloroform by weight to dissolve, blow in nitrogen, add 6 parts of 2-ethylhexyl methacrylic anhydride, and heat to 70°C, Incubate for 2 hours, cool, add dropwise to 150 parts of ethanol, filter with suction, heat up to 40°C, and vacuum dry for 15 hours to obtain intermediate materials; add 7 parts of itaconic acid to 40 parts of oxalyl chloride in parts by weight, heat up to 90°C, keep warm 3h, suction filtration, add the filter cake to 40 parts of ether and soak for 22h...
Embodiment 2
[0044] An absorbable medical suture thread with good compatibility. Its raw materials by weight include: 20 parts of polyglycolide, 20 parts of modified polylactic acid, 50 parts of polylactic acid, 20 parts of hyaluronic acid, and 5 parts of methyl methacrylate. Parts, 6 parts of epoxidized soybean oil, 2 parts of tea tree oil, 5 parts of polycaprolactone, 0.1 part of calcium stearate, 30 parts of ethanol;
[0045] Among them, the preparation method of modified polylactic acid is as follows: add 4 parts of polylactic acid to 10 parts of chloroform by weight to dissolve, blow in nitrogen, add 13 parts of 2-ethylhexyl methacrylic anhydride, and heat to 50°C, Incubate for 4 hours, cool, add dropwise to 100 parts of ethanol, filter with suction, heat up to 60°C, and vacuum dry for 10 hours to obtain intermediate materials; add 11 parts of itaconic acid to 30 parts of oxalyl chloride in parts by weight, heat up to 110°C, keep warm 2h, suction filtration, add the filter cake to 60 par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com