Synthesis method of pentaerythritol
A pentaerythritol and synthesis method technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of low added value of sodium salt, low content of pentaerythritol, large amount of manual labor, etc., to reduce sodium The process of salt recovery, the effect of reducing the generation of three wastes and reducing the consumption of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
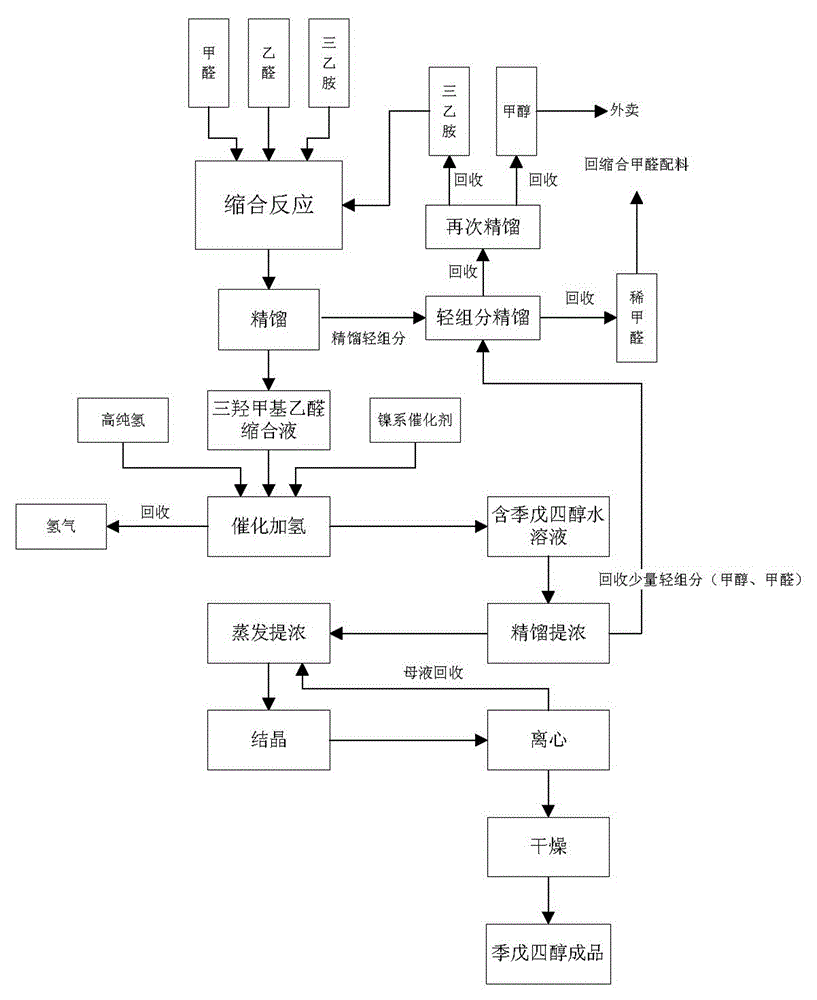
[0015] Such as figure 1 As shown, the present invention provides a process for synthesizing pentaerythritol by catalytic hydrogenation. The basic process steps are as follows: 1) put the prepared formaldehyde, acetaldehyde, and triethylamine into the condensation tank as required to carry out aldol condensation reaction Generate trimethylol acetaldehyde condensation liquid; 2) Quickly carry out rectification and separation after the condensation reaction, so that formaldehyde exists in triethylamine in the shortest possible time, avoiding the formation of side reactions, and rectifying formaldehyde and triethylamine and a small amount of methanol, formaldehyde and triethylamine are rectified again, and formaldehyde and triethylamine are separated, and the formaldehyde and triethylamine return to the condensation reaction to participate in the reaction again; 3) trimethylol acetaldehyde aqueous solution obtained after rectification Catalytic hydrogenation, using nickel-based ca...
example 2
[0018] Prepare formaldehyde, acetaldehyde and triethylamine according to the molar ratio of 5.5:1:1, first add formaldehyde to the condensation kettle, then drop triethylamine step by step, adjust the pH=8.0-8.2, and then drop acetaldehyde step by step During the reaction process, the pH is controlled at 8~9, the temperature is controlled at 28-30°C, and the reaction time is 1 hour. After the reaction results, the condensation liquid is pumped into the rectification tower, and the light components are rectified to obtain trimethylol ethyl alcohol. The aqueous solution of aldehyde is passed into the hydrogenation tower with high-purity hydrogen (purity above 99.9%), and the Raney nickel RTH-311 catalyst is used for catalytic hydrogenation reaction to obtain a pentaerythritol aqueous solution. A small amount of methanol is rectified from this aqueous solution to obtain concentrated The final pentaerythritol aqueous solution is evaporated, crystallized, centrifuged, and dried to o...
example 3
[0020] Prepare formaldehyde, acetaldehyde, and triethylamine according to the molar ratio of 5.8:1:1, first add formaldehyde to the condensation kettle, drop triethylamine step by step, adjust the pH=8.0, then drop acetaldehyde step by step, and react During the process, the pH is controlled at 8~9, the temperature is controlled at 28°C, and the reaction time is 1 hour. After the reaction results, the condensation liquid is pumped into the rectification tower, and the light components are rectified to obtain an aqueous solution of trimethylolacetaldehyde. Pass high-purity hydrogen (above 99.9% purity) into the hydrogenation tower, and use Raney nickel RTH-311 catalyst for catalytic hydrogenation reaction to obtain pentaerythritol aqueous solution. A small amount of methanol is rectified from this aqueous solution to obtain concentrated pentaerythritol aqueous solution , the solution was evaporated, crystallized, centrifuged, and dried to obtain the finished product of pentaeryt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com