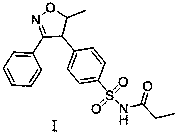
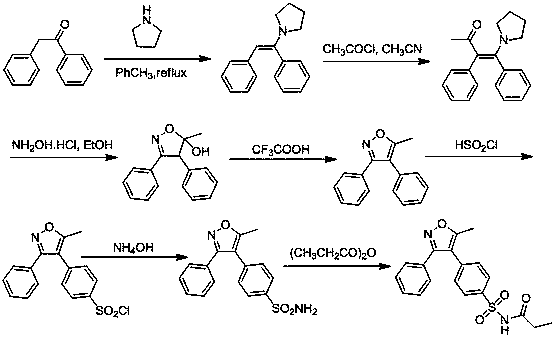
A kind of method for preparing parecoxib
A parecoxib and dioxo technology, applied in organic chemistry and other directions, can solve the problems of unstable storage, high equipment requirements, and very high safety requirements, achieve high product yield and purity, easy post-processing operations, Reaction conditions require low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] F, the preparation of parecoxib:
[0051] The N-(4-(5-hydroxyl-5-methyl-3-phenyl-4,5-dihydroisoxazol-4-yl)phenylsulfonyl)propionamide prepared in step E was prepared in -5 Add it into an acidic solvent with a solid-to-liquid volume ratio of 1:2 to 1:3 under ice-bath conditions at ~5°C, and keep stirring at a temperature of 0~30°C for 4~12 hours to obtain the target product parecoxib.
[0052] The organic solvent described in step A is one of benzene, toluene, tetrahydrofuran, ethyl acetate, dichloromethane, chloroform or acetonitrile.
[0053] The Lewis acid catalyst described in step A is one of aluminum trichloride, titanium tetrachloride, boron trifluoride, ferric chloride, zinc chloride, niobium pentoxide, trisulphonate, p-toluenesulfonic acid species, the amount added is 5-20% of the molar weight of the raw material.
[0054] The sulfonating reagent described in step B is a kind of in chlorosulfonic acid, concentrated sulfuric acid, oleum, sulfur trioxide.
[00...
Embodiment 1
[0076] Preparation of 1,2-diphenylbutane-1,3-dione (III)
[0077] Weigh 4.0 g (20.4 mmol, 1e.q) of 3-oxo-2-phenylbutyryl chloride and dissolve it in 40 mL of benzene solution, add 150 mg of aluminum trichloride solid, heat and reflux for 12 hours, until TLC shows that the raw material After the reaction was complete, stop the reaction, wash once with saturated aqueous sodium bicarbonate solution, wash once with saturated brine, dry the organic phase with anhydrous sodium sulfate, and spin dry the solvent to obtain about 4.0 g of solid product with a yield of 84%. 1 H NMR (500 MHz, CDCl 3 ): δ = 7.6-7.1 (m, 10H), 5.40 (s, 1H), 2.26 (s, 3H).
Embodiment 2
[0079] Preparation of 4-(1,3-dioxo-1-phenylbut-2-yl)benzene-1-sulfonyl chloride (IV)
[0080] Weigh 2.4 grams of 1,2-diphenylbutane-1,3-dione, add the above raw materials in batches to 5 ml of chlorosulfonic acid under stirring under ice bath conditions, and keep stirring at 0°C After 6 hours, after stopping the reaction, the reaction mixture was poured into 10 g of crushed ice, and a white solid precipitated out. The white solid was collected by filtration, washed with a small amount of ice water, and the dried white solid product was 3.1 g, with a yield of 91%. 1 H NMR (500 MHz, CDCl 3 ): δ = 7.70 (dd, J= 8.1 Hz, 2H), 7.60(dd, J = 8.1 Hz, 2H), 7.53-7.41 (m, 5H), 5.48 (s, 1H), 2.26 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com