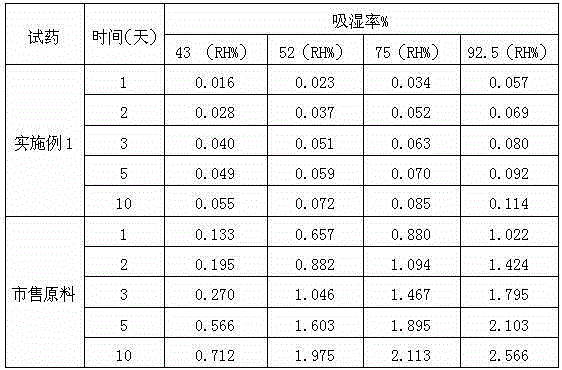
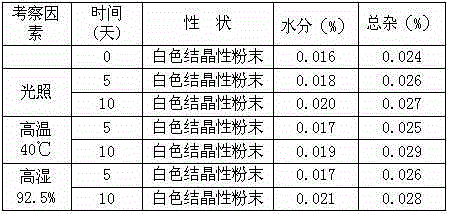
Medicine hydrochloric acid fasudil composite dry suspension for treating ischemic cerebrovascular disease
A technology for fasudil hydrochloride and cerebrovascular disease, applied in the field of medicine, can solve the problems of solubility difference of crystalline drugs, large surface free energy per unit, easy hydration of the surface between particles, etc., and achieves good fluidity and bioavailability. High, safe and reliable effect in clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of Fasudil Hydrochloride Crystals
[0028] (1) Dissolve fasudil hydrochloride in a mixed solvent of methanol and propyl acetate, the required amount of solvent per gram of fasudil hydrochloride is 120ml, and the volume ratio of methanol to propyl acetate is 5:3;
[0029] (2) After heating to 30°C to dissolve, add seed crystals after cooling to room temperature;
[0030] (3) Cool to below -5°C, stir and crystallize, the crystallization temperature is -10°C, filter, dry, collect crystals to obtain fasudil hydrochloride crystals.
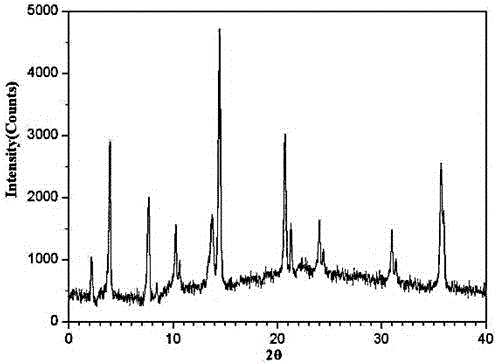
[0031] The prepared Fasudil hydrochloride crystals use Cu-Kα rays to measure the X-ray powder diffraction pattern obtained as figure 1 shown.
Embodiment 2
[0032] Example 2: The preparation of Fasudil hydrochloride dry suspension, the steps are as follows:
[0033] Prescription: Fasudil hydrochloride crystalline form compound that the embodiment 1 of 3 parts by weight makes, the mannitol of 18 parts by weight, the sodium alginate of 4 parts by weight, the locust bean gum of 1.5 parts by weight, the stearin of 0.9 parts by weight Sodium alcohol sulfonate, 0.25 parts by weight of stevia, 95% ethanol by weight.
[0034] Preparation:
[0035] (1) Processing of raw and auxiliary materials: sieve Fasudil hydrochloride through 100 mesh;
[0036] (2) Weighing: Weighing according to the prescription;
[0037] (3) Granulation: Add fasudil hydrochloride, mannitol, sodium alginate, locust bean gum, sodium stearyl sulfonate and stevia into the wet mixing granulator, dry mix for 10 minutes, and mix 95 % ethanol is added to the wet mixing granulator for wet mixing and cutting, and 18 mesh granulation is selected to make soft materials;
...
Embodiment 3
[0041] Example 3: The preparation of Fasudil hydrochloride dry suspension, the steps are as follows:
[0042] Prescription: Fasudil hydrochloride crystalline form compound that the embodiment 1 of 3 parts by weight makes, the mannitol of 19 parts by weight, the sodium alginate of 5 parts by weight, the locust bean gum of 1.6 parts by weight, the stearin of 0.95 parts by weight Sodium alcohol sulfonate, the stevioside of 0.3 weight part, the 95% ethanol of 9.5 weight part.
[0043] Preparation:
[0044] (1) Processing of raw and auxiliary materials: sieve Fasudil hydrochloride through 100 mesh;
[0045] (2) Weighing: Weighing according to the prescription;
[0046] (3) Granulation: Add fasudil hydrochloride, mannitol, sodium alginate, locust bean gum, sodium stearyl sulfonate and stevia into the wet mixing granulator, dry mix for 10 minutes, and mix 95 % ethanol is added to the wet mixing granulator for wet mixing and cutting, and 18 mesh granulation is selected to make so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com