Beta-carbonyl enamine compounds and application for preparing phytopathogen antibacterial agent
A technology for plant pathogenic bacteria and carbonyl enamine, which is applied in the field of preparation of plant pathogenic bacteria antibacterial agents, the field of β-carbonyl enamine compounds, can solve problems such as drug resistance of diseased bacteria, and achieves high efficacy, simple post-processing and synthesis. short step effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The synthetic route of the preparation β-carbonyl enamine compound established by the present invention is as follows:
[0044] (1) Synthesis of β-1,3-diketone
[0045] Substituted acetophenone and NaNH 2 Reflux in ethyl acetate for 10-15 hours, that is, substituted acetophenone undergoes Claisen condensation reaction with ethyl acetate under the catalysis of sodium amide, and β-1,3-diketone can be prepared.
[0046]
[0047] (2) Synthesis of β-carbonyl enamine compounds
[0048] β-1,3-diketone and CH 3 COONH 4 Refluxing in methanol solution for 1 to 3 hours, a typical nucleophilic addition reaction unique to ketone compounds that are first added and then eliminated occurs, and the target compound β-carbonyl enamine can be synthesized.
[0049]
[0050] The specific operation steps of synthesizing different types of β-carbonyl enamine compounds are as follows:
[0051] 1. Compound 1 is the synthesis of 4-phenyl-4-oxo-2-butene-2-amine
[0052] (1) Add 6.06g (...
example 1
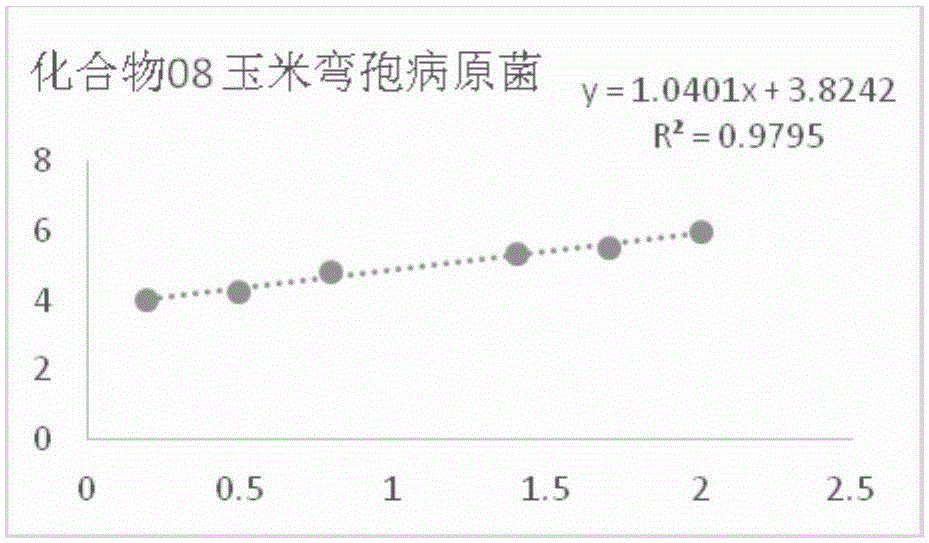
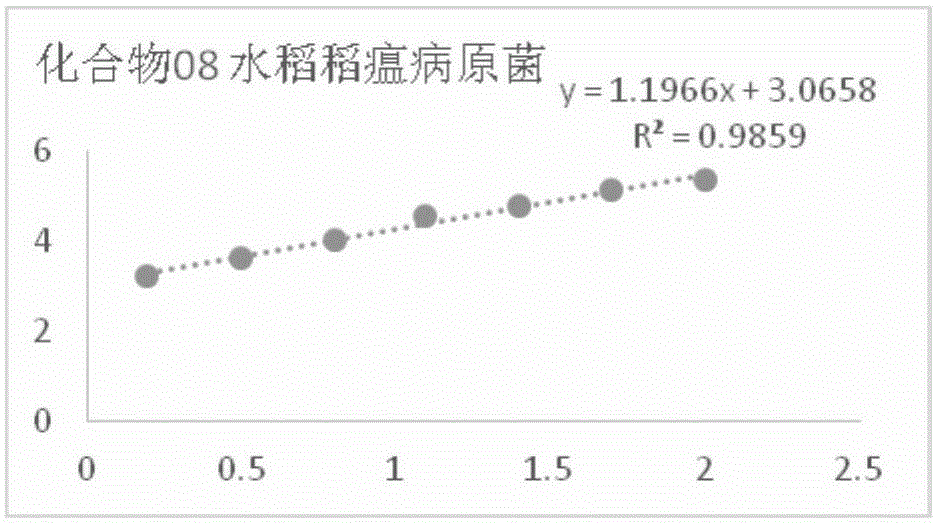
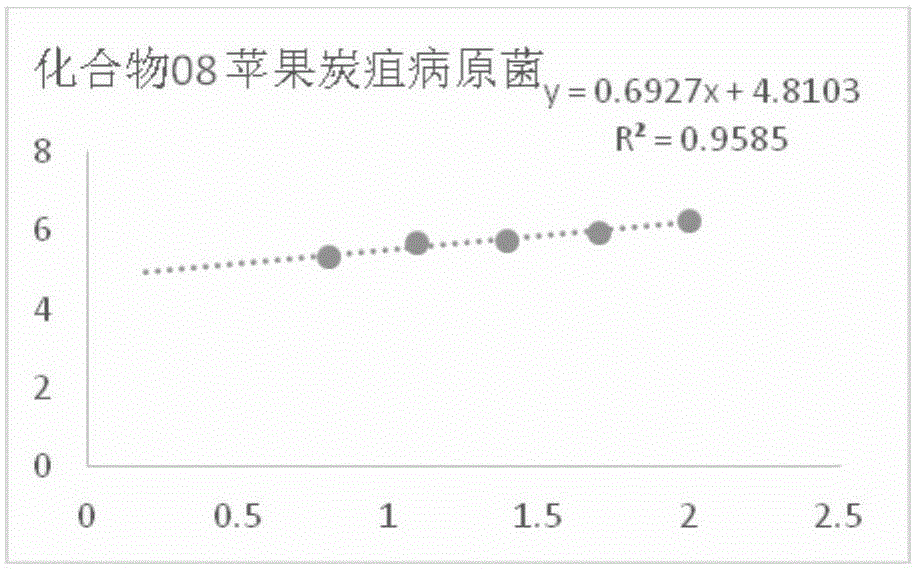
[0093] In vitro bacteriostatic activity of example 1 compound 8 to rice blast pathogenic fungus
[0094] Accurately weigh 5 mg of compound 8 in a 20 mL vial, inject 0.5 mL of DMSO and 9.5 mL of sterile water into it, and make a 5% DMSO solution of the sample to be tested; Make 100mL culture medium containing the sample to be tested, and pour it into a sterile petri dish to make a flat culture medium with medicine. The same amount of solvent, namely 5% DMSO, was used as a blank control, and the commercially available antibacterial drug kresoxim-methyl was used as a positive control.
[0095] Use a hole puncher with a diameter of 5 mm to take out the rice blast pathogen bacterial cake that grows vigorously on the edge of the colony, transfer it to the above-mentioned flat culture medium with an inoculation needle, and paste it on the culture medium with the mycelium side facing down. Blocks were placed in the center in a triangular shape, covered and placed in a constant temper...
example 2
[0098] In vitro bacteriostatic activity of example 2 compound 14 to watermelon wilt pathogenic fungus
[0099] Accurately weigh 5 mg of compound 14 in a 20 mL vial, inject 0.5 mL of DMSO and 9.5 mL of sterile water into it, and make a 5% DMSO solution of the sample to be tested; Make 100mL culture medium containing the sample to be tested, and pour it into a sterile petri dish to make a flat culture medium with medicine. The same amount of solvent, namely 5% DMSO, was used as a blank control, and the commercially available antibacterial drug kresoxim-methyl was used as a positive control.
[0100] Use a hole puncher with a diameter of 5mm to take out the bacteria cake of watermelon wilt pathogen growing vigorously at the edge of the colony, transfer it to the above-mentioned flat culture medium with an inoculation needle, and paste it on the culture medium with the mycelium side facing down, 3 pieces per dish , placed in the center in a triangular shape, covered and cultured ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com