Ultrafine powder of macrolide drug and preparation method for ultrafine powder
A macrolide and ultrafine powder technology, applied in the field of medicine, can solve the problems of supercritical fluid state temperature, pressure, difficult configuration of production equipment, large difference in product size, etc., and achieve parallel production process. Good, stable powder morphology, good uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
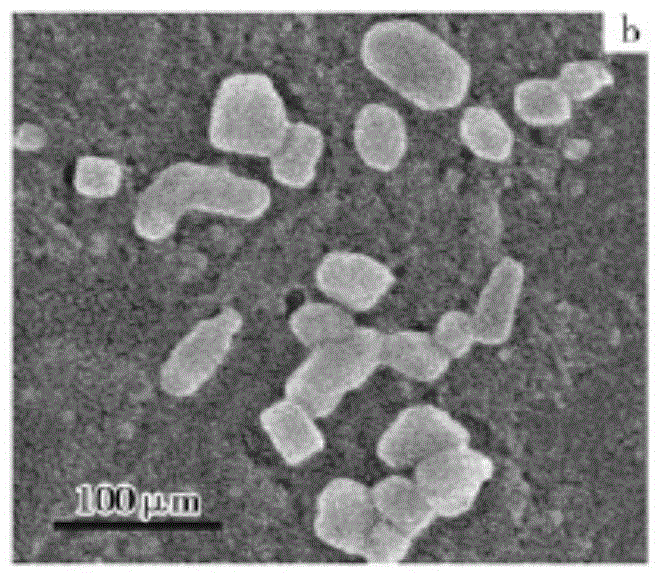
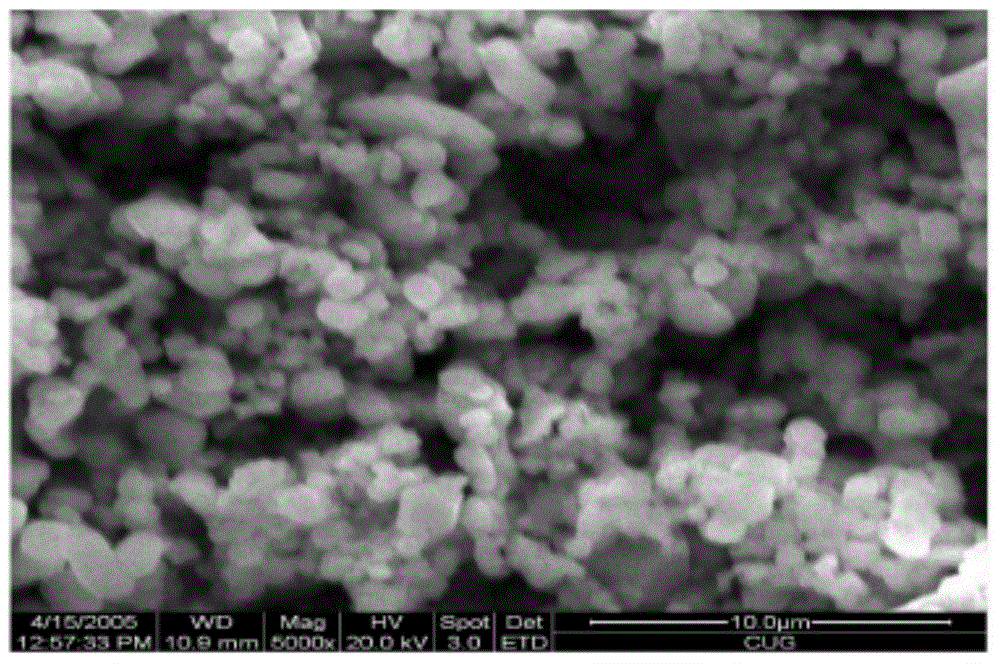
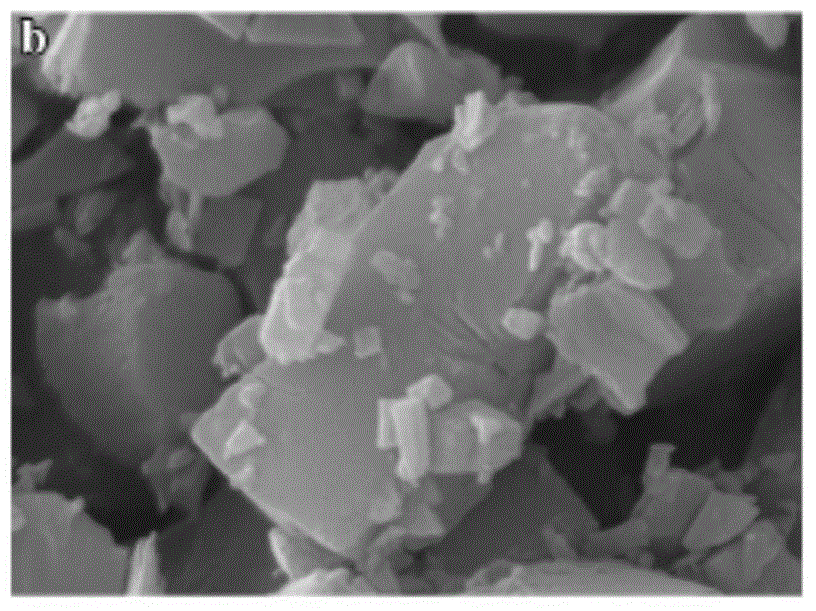
[0059] Azithromycin raw material 10g, add 30ml acetone, heat to dissolve, add water 5ml, ice bath cooling, 20kHz, 150W ultrasonic, obtain white crystal; Collect, wash, dry and obtain one-dimensional ultrafine powder (US FEISirion200 field scanning electron microscope, attached Figure 7 , 8 ).
Embodiment 2
[0061] Add 10g of roxithromycin raw material, add 25ml of acetone, heat to dissolve, add 10ml of water, cool in an ice bath, 20kHz, 150W ultrasonic, to obtain white crystals; collect, wash and dry to obtain ultrafine powder.
Embodiment 3
[0063] Add 20g of erythromycin raw material, add 50ml of acetone, heat to dissolve, add 16ml of water, cool down in ice bath, 20kHz, 200W ultrasonic to obtain white crystals; collect, wash and dry to obtain ultrafine powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com