A kind of pyrididine oxime ester compound and its preparation method and application
A technology of pyrididine and compound, which is applied in the field of pyrididine oxime ester compound and its preparation, and achieves the effects of cheap raw materials, good killing activity and simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
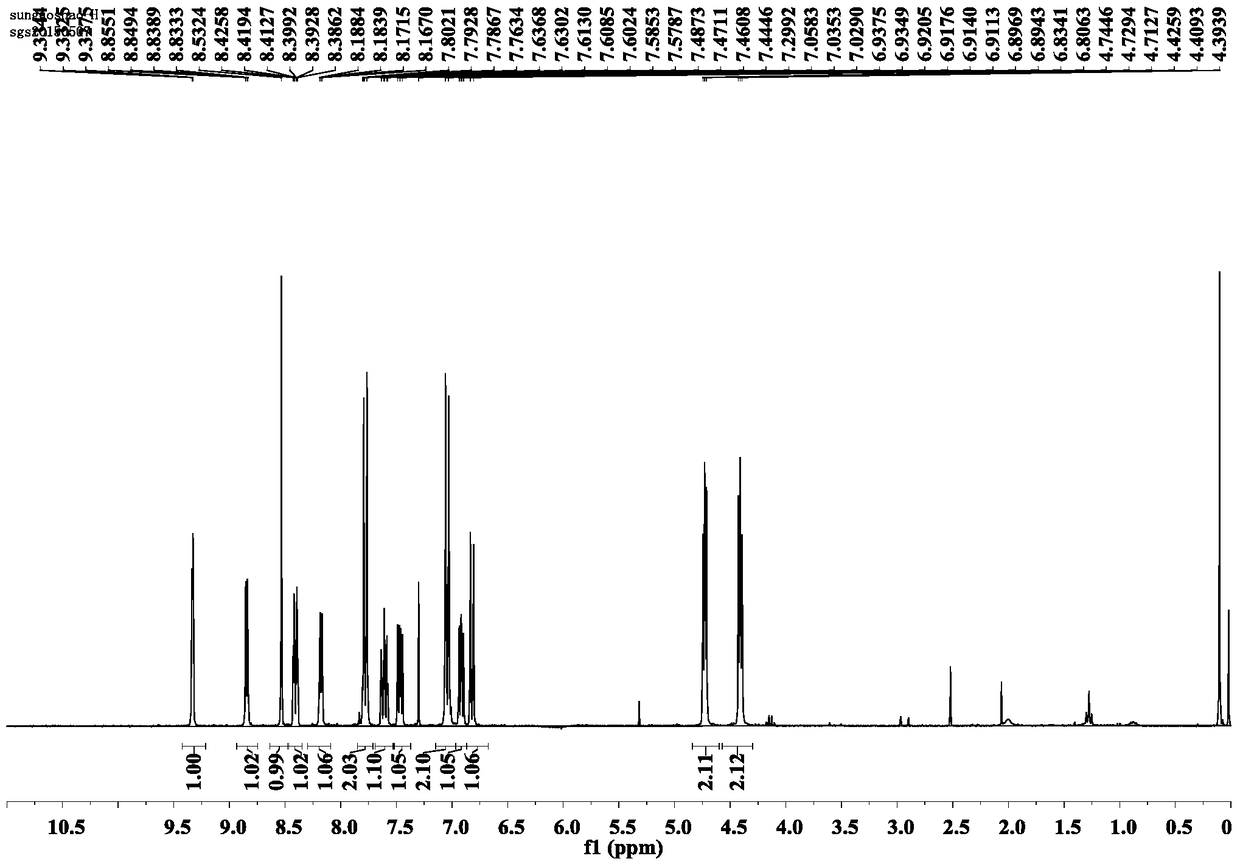
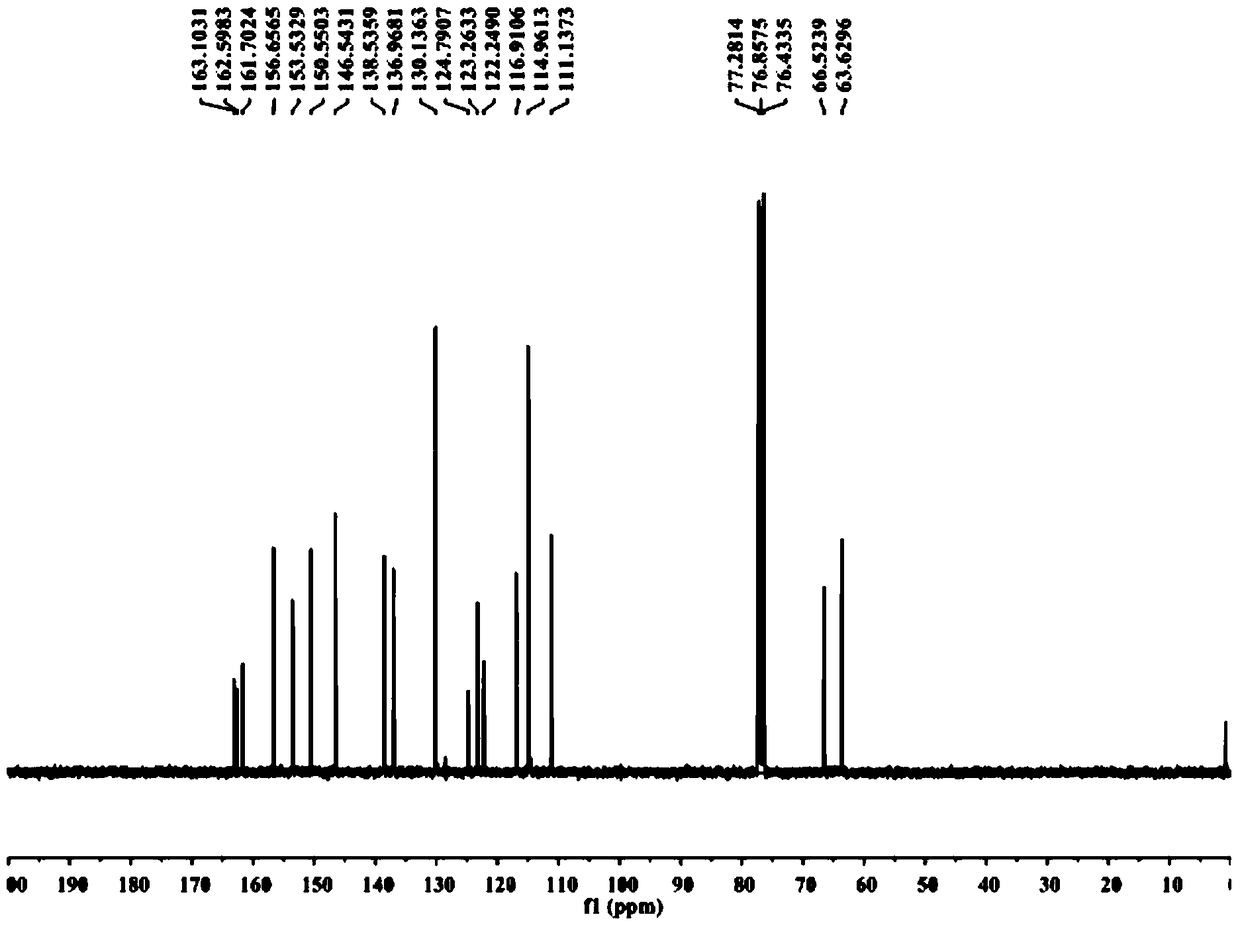
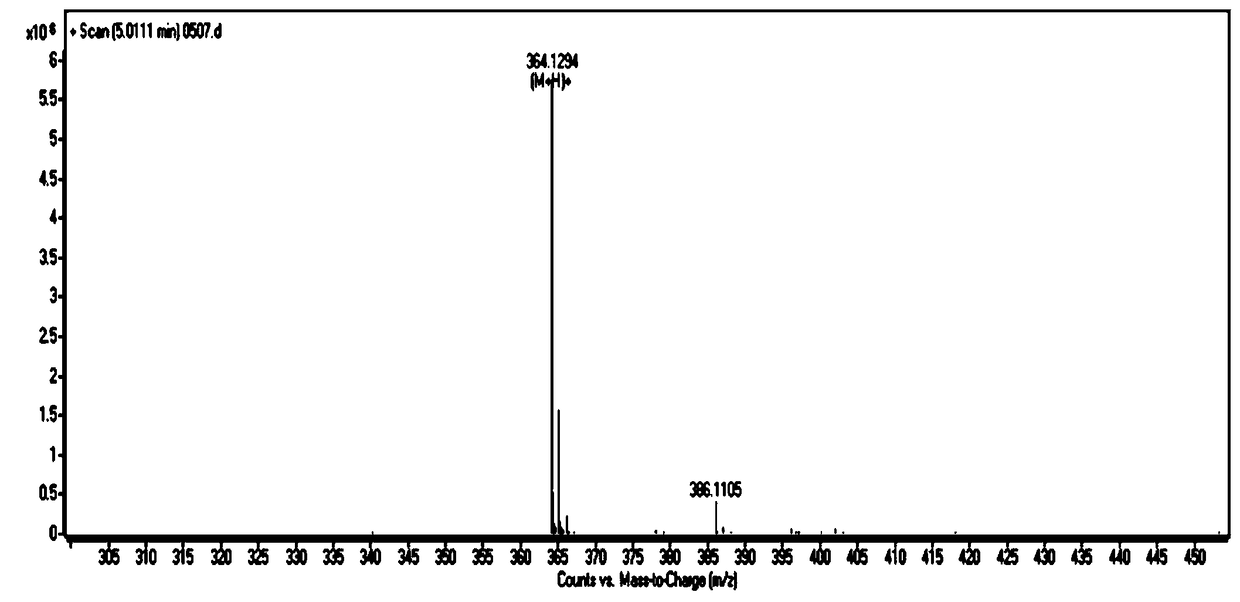
[0031] Example 1: Preparation and structure identification of CAU-PD-19 (R=3-pyridyl) in Pyridoxine oxime ester compound (CAU-PD):
[0032]
[0033] Add 10g (82.0mmol) p-hydroxybenzaldehyde to a 250mL round bottom flask, dissolve in 30mL DMF, then add 22.6g (163.8mmol) potassium carbonate and 12.3g (98.3mmol) 2-bromoethanol, and react at 100℃ for 12h; TLC (V(Petroleum ether):V(ethyl acetate)=5:1) After confirming that the reaction of the raw materials is complete, cool to room temperature, pour the reaction solution into water, extract 3 times with 200mL ethyl acetate, dry with anhydrous sodium sulfate, and concentrate Column chromatography (V (petroleum ether): V (ethyl acetate) = 10:1) separated to obtain 11.3 g of a white product with a yield of 83%.
[0034] (2) Add 5.0 g (30.1 mmol) of the product obtained in step (1) into a 500 mL round bottom flask, dissolve it with 40 mL of dry DMF, add 1.4 g (36.1 mmol) of sodium hydride and stir for half an hour under ice bath, then add 3...
Embodiment 2
[0052] Example 2: Preparation method of CAU-PD-19 (R=phenyl) preparation in Pyridoxine oxime ester compound (CAU-PD).
[0053] (1) Emulsifiable concentrate: add 1-10g compound CAU-PD-19, 5-15g emulsifier, 0.1-1g penetrant in a 100mL volumetric flask, and then use solvent (such as toluene, xylene, etc.) to make CAU-PD. -19 EC with a mass fraction of 1-10%.
[0054] Other emulsifiable concentrates with the general formula of CAU-PD series compounds can be prepared according to the above methods.
[0055] (2) Wettable powder: take 15-50g compound CAU-PD-19, 10-20g surfactant, 30-75g white carbon black, after mixing and pulverizing, the mass fraction of CAU-PD-19 is 15-50% Wettable powder.
[0056] Other wettable powders of the series of compounds with the general formula CAU-PD can be prepared according to the above method.
Embodiment 3
[0057] Example 3: Determination of insecticidal and ovoicidal activity of a series of compounds with the general formula CAU-PD.
[0058] (1) Test for killing peach aphid: immerse the leaves with green peach aphid in the chemical solution for 5 seconds, and record the number of green peach aphid after drying and put them in a petri dish with moisturizing filter paper. After the petri dish is covered, put 25± In a light incubator at 1°C. Each drug treated more than 30 heads, check the results after 24-48 hours, calculate the corrected mortality (%), and compare with the control drug (pyriproxyfen) to determine the toxicity of the drug. Table 3 shows the insecticidal activity data of the series of compounds with the general formula CAU-PD against Myzus persicae.
[0059] Table 3: Insecticidal activity of CAU-PD series compounds against Myzus persicae
[0060]
[0061] Note: The control agent pyriproxyfen and the sample are both 600mg / L
[0062] From the results in Table 3, the compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com