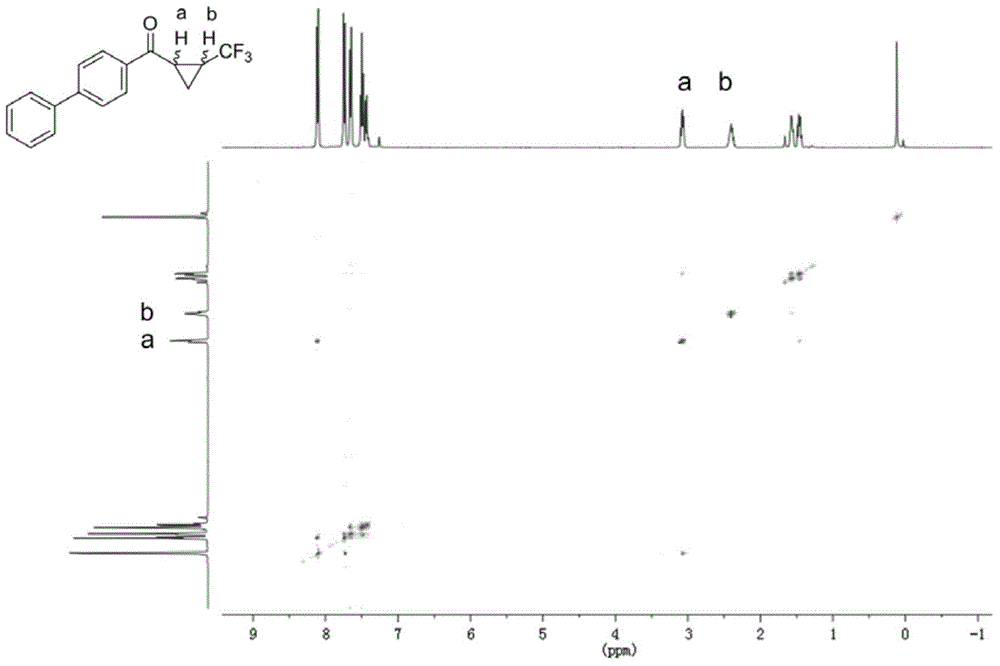
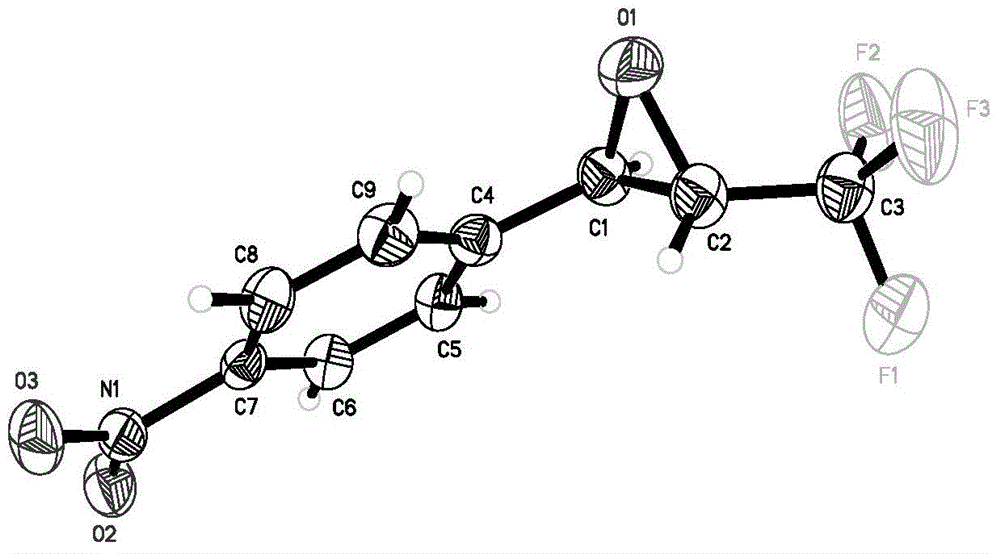
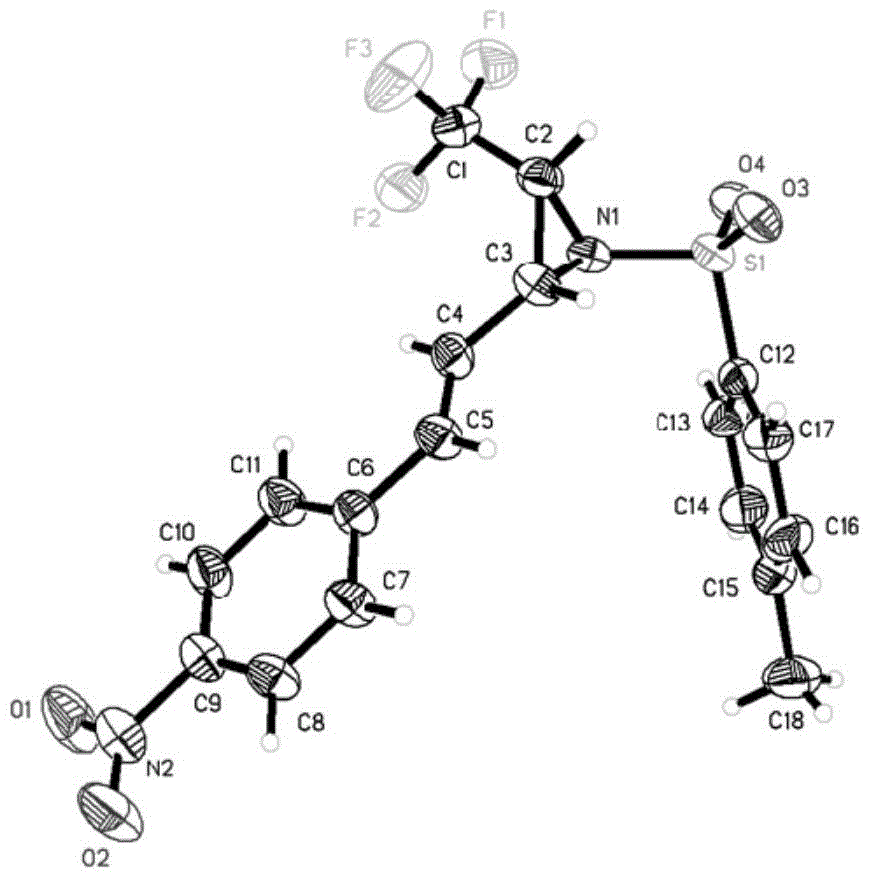
Fluorine-containing three-membered ring compound, preparation method of fluorine-containing three-membered ring compound and preparation method of fluoroalkyl sulfonium salt
A compound and three-membered ring technology, applied in the field of fluorine-containing three-membered ring compounds, can solve the problems of complicated preparation method steps, difficult to obtain raw materials, complicated operation steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1-1
[0173]
[0174] Add paraformaldehyde (7.5g, 250 / nmmol), TAMA (nitromethylaniline trifluoroacetic acid 11.05g, 50mmol) into the 250mL three-necked flask, stirrer, add THF (50mL) under nitrogen protection after pumping, as The aryl ethyl ketone substrate represented by Formula 5 (50 mmol) was heated to reflux. After reacting for 10 hours, the stirring was stopped, the solution was returned to room temperature, the solvent was evaporated under reduced pressure, and the obtained paste was dissolved in 150 mL of ethyl acetate. After washing with water (100 mL×2) in a separatory funnel to remove a large amount of TAMA salt, the amine contained in the solution was removed with 1M HCl. Wash once more with 100 mL of water, then wash with saturated sodium bicarbonate solution until the solution becomes neutral, wash with saturated brine, and dry over anhydrous sodium sulfate. Petroleum ether: ethyl acetate (50:1) column chromatography to obtain the compound shown in formula 4.
[0...
Embodiment 1-1-2
[0204]
[0205] A substituted arylaldehyde (20mmol, 1.0equiv), p-toluenesulfonamide (20mmol, 3.424g, 1.0equiv) and tetraethylorthosilicate (80mmol, 16.82g, 4.0equiv) as shown in Formula 6 were mixed In a 100mL round bottom flask. Install a water separator and a condenser, and evaporate most of the ethanol produced in the reaction at 160°C. Situation 1: If crystals are precipitated in the system, stop heating and stirring, wait to cool to room temperature, wash with a small amount of mixed solvent (the volume ratio of ethyl acetate to petroleum ether is about 1:10), filter with suction, and wash with ethanol The cake was dried two to three times, and the obtained solid was weighed to calculate the yield after vacuum drying. Situation 2: There is still no solid precipitation after a large amount of ethanol is evaporated, and if there is crystal precipitation after cooling, it should be treated as situation 1. Situation 3: If there is still no solid precipitation after cooli...
Embodiment 1-1 3
[0238] The synthesis of embodiment 1-1 trifluoroethyl diphenylsulfonium salt
[0239]
[0240] Mix trifluoroethyl trifluoromethanesulfonate (9.28g, 40mmol) and diphenyl sulfide (37.2g, 0.2mol) in a 100mL sealed tube, heat in an oil bath at 150°C for 24h, stop heating, and cool naturally The sealed tube was opened, and a solid precipitated out. The solid was washed with ether, filtered with suction to obtain a white product, recrystallized with tetrahydrofuran, and dried to obtain 26.78 g of white crystals, with a yield of 80% and a purity of 99.9% by HPLC.
[0241]
[0242] 1 HNMR (400MHz, acetone-d 6 )δ8.36(d, J=7.7Hz, 4H), 7.93(t, J=7.7Hz, 2H), 7.84(t, J=7.7Hz, 4H), 5.75(q, J=8.8Hz, 2H) ; 19 FNMR (376MHz, acetone-d 6 )δ-60.80(t,J=8.8Hz,3F),-78.50(s,3F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com