Esomeprazole magnesium enteric-coated tablets and preparation method thereof
A technology of esomeprazole magnesium enteric-coated tablets and esomeprazole magnesium applied in the field of pharmaceutical preparations to achieve the effects of good reproducibility and stability, large production capacity and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
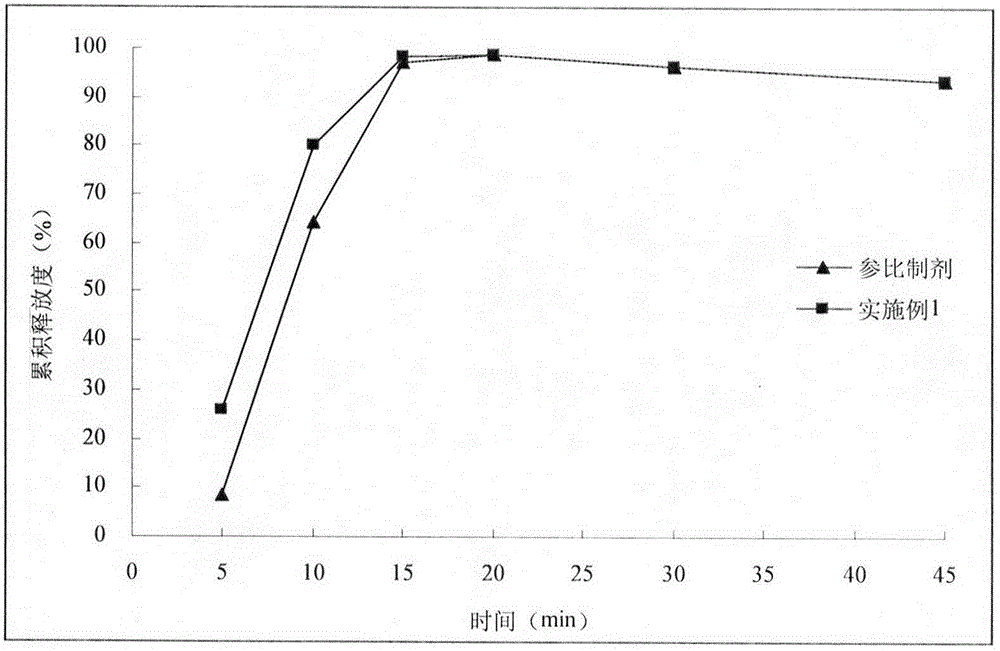
Embodiment 1
[0019] Prescription composition:
[0020]
[0021] Preparation Process:
[0022] (1) Preparation of tablet core
[0023] Weigh the main ingredient, pregelatinized starch, lactose, and croscarmellose sodium according to the prescription quantity, mix well, use an appropriate amount of PVPk30 aqueous solution as a binder to make a soft material, granulate, dry, granulate, and add the prescription quantity Magnesium stearate, mixed evenly and compressed into tablet cores.
[0024] (2) Isolation layer coating
[0025] Add HPMCE5 to an appropriate amount of purified water, stir to dissolve, add talcum powder to prepare a suspension solution, sieve, and stir for later use. Place the tablet cores in the coating pan and wrap the isolation layer.
[0026] (3) Enteric layer coating
[0027] Disperse Acryl-EZE 93F19255 in an appropriate amount of purified water to prepare a suspension solution with a solid content of 20%, sieve, and stir for later use. Place the isolation tablet...
Embodiment 1-4
[0028] The assay method of embodiment 1-4 release degree:
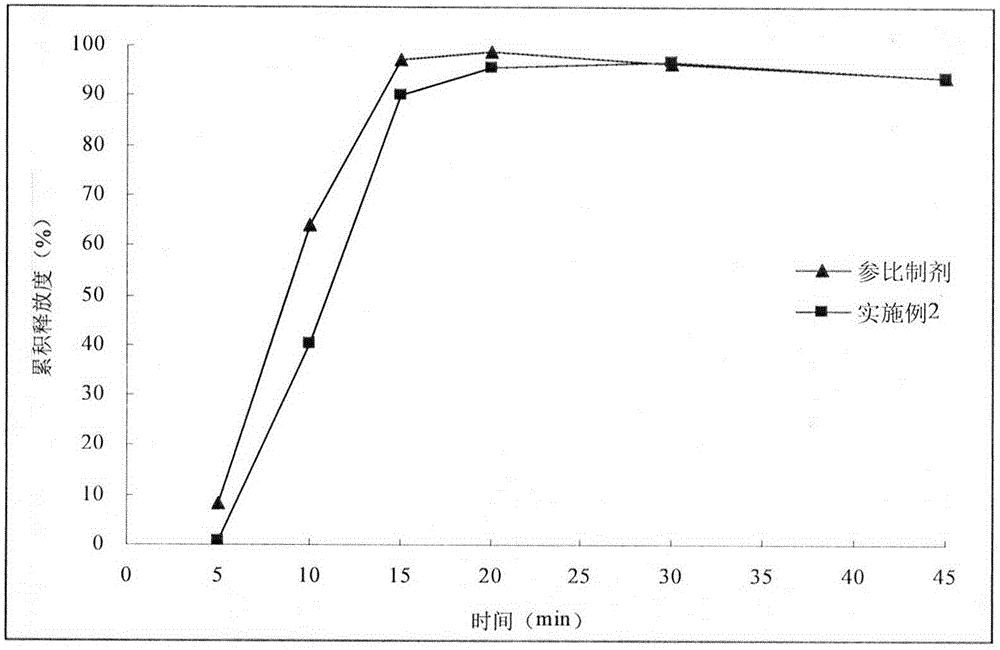
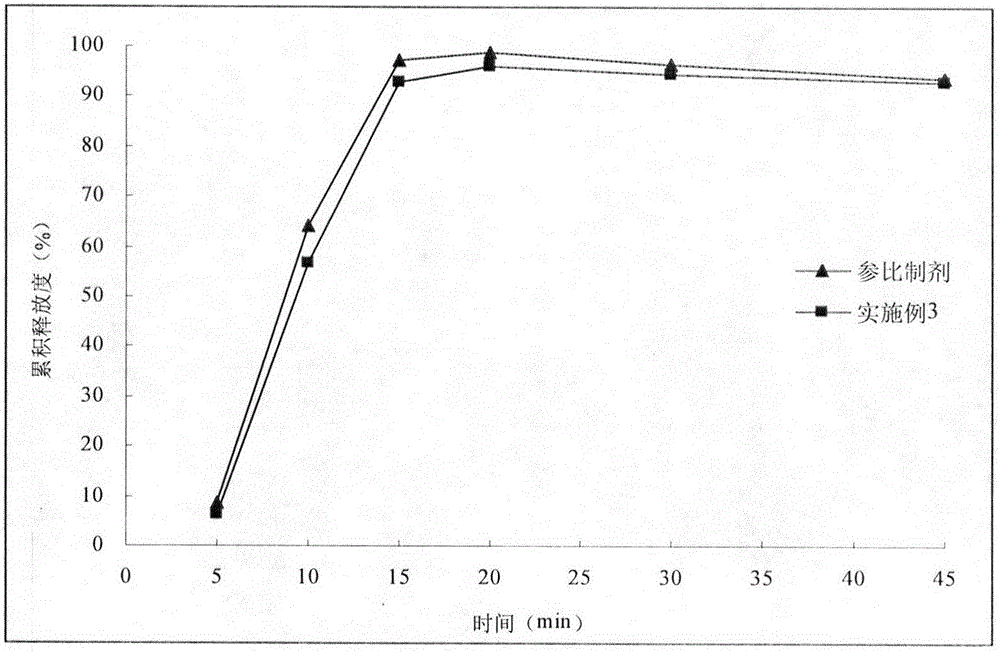
[0029] With reference to the import standard of Esomeprazole Magnesium Enteric-coated Tablets, the second method of release measurement method was adopted, and the preparation was tolerated in 0.1mol / L hydrochloric acid solution for 2 hours, and then lye was added to prepare a phosphate buffer solution with a pH of 6.8. , continue to release for 45 minutes. After 2 hours of hydrochloric acid medium resistance, the sampling time points are 5min, 10min, 15min, 20min, 30min, 45min. Sampling 5mL at each time point was filtered with a 0.45μm filter membrane, and an equal amount of fresh medium was added immediately, and the filtrate was mixed with an alkalinizing agent in equal proportions, and 20μL of the mixed solution was injected into a high-performance liquid chromatograph to determine esomemera The peak area of azole was used to calculate the cumulative release. Select commercially available esomeprazole magnesiu...
Embodiment 2
[0032] Prescription composition:
[0033]
[0034]
[0035] Preparation Process:
[0036] (1) Preparation of tablet core
[0037] Weigh the main ingredient, pregelatinized starch, lactose, and croscarmellose sodium according to the prescription quantity, mix well, use an appropriate amount of PVPk30 aqueous solution as a binder to make a soft material, granulate, dry, granulate, and add the prescription quantity Magnesium stearate, mixed evenly and compressed into tablet cores.
[0038] (2) Isolation layer coating
[0039] Add HPMCE5 to an appropriate amount of purified water, stir to dissolve, add talcum powder to prepare a suspension solution, sieve, and stir for later use. Place the tablet cores in the coating pan and wrap the isolation layer.
[0040] (3) Enteric layer coating
[0041] Take the prescribed amount of purified water into the liquid mixing container, add PEG6000, stir until dissolved, add talcum powder and stir evenly; add it to Eudragit L30D-55, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com