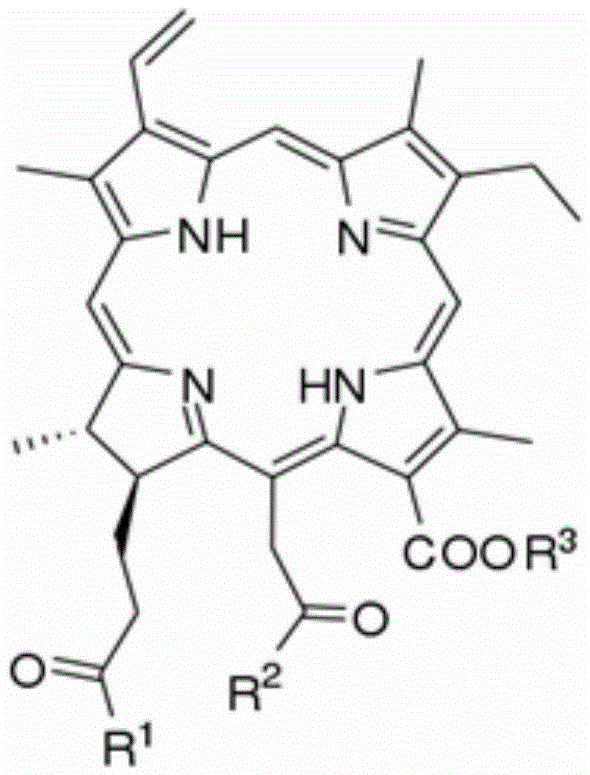
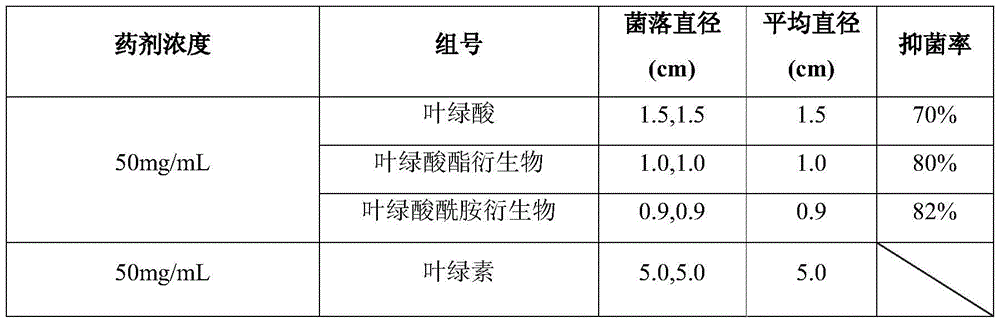
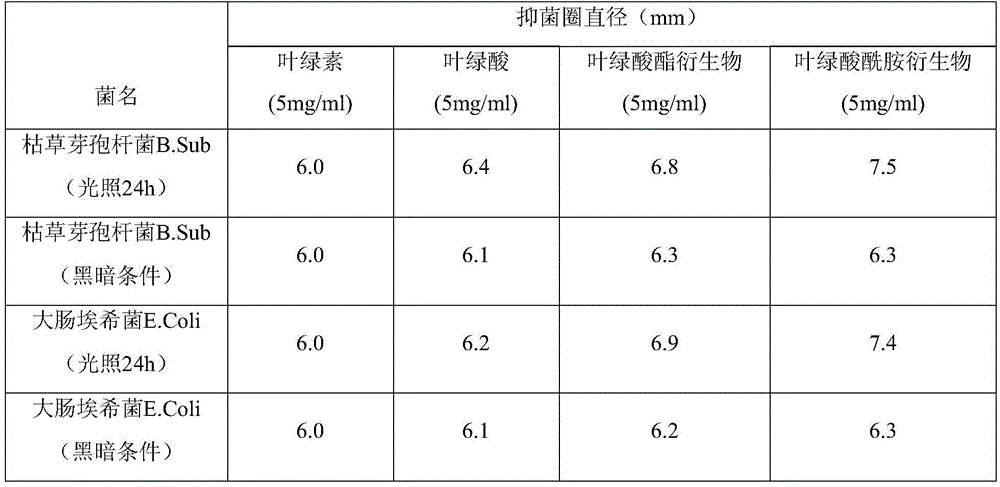
Chlorophyllin derivative and preparation method thereof and application of chlorophyllin derivative to bacteriostasis and insect disinfestation
A technology of chlorophyllin and derivatives, applied in the application of chlorophyllin derivatives, photosensitive insecticides, its preparation method and as a photosensitive antibacterial agent, can solve the problems of harming human health and the environment, reducing the efficacy of antibiotics, Toxic and side effects and other problems, to achieve the effect of high quantum yield, high photosensitivity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0024] Specific embodiment 1: the synthesis of chlorophyllin
[0025] (1) Weigh 1g of silkworm excrement chlorophyll primary extract, add 10mL of acetone to dissolve, stir for 30min, add 20ml of concentrated NaOH solution, place in a constant temperature water bath at 50°C and stir for 2h, after it is fully hydrolyzed, let stand for 2h;
[0026] (2) Suction filter the precipitate obtained from step (1), dissolve it in 30 mL of water, and extract three times with 30 mL of petroleum ether; then use 18% HCl to adjust the pH of the solution to about 1, leave it at room temperature for 2 hours, and there is precipitation Precipitate, filter to collect the precipitate, wash the precipitate with distilled water, and dry to obtain a dark green solid. The content of chlorophyllin in the product can be determined to be 62.60% through high-performance liquid chromatography analysis and comparison with standard products.
specific Embodiment 2
[0027] Specific Example 2: Synthesis of Chlorophyllin Derivatives
[0028] (1) Weigh 2g of silkworm excrement chlorophyll primary extract, add 25mL of acetone to dissolve, stir for 30min, add 40ml of concentrated NaOH solution, place in a constant temperature water bath at 50°C and stir for 2h, after it is fully hydrolyzed, let stand for 2h;
[0029] (2) Suction filter the precipitate obtained from step (1), dissolve it in 60 mL of water, and extract three times with petroleum ether; then use 18% HCl to adjust the pH of the solution to about 1, leave it at room temperature for 2 hours, and precipitate , filter to get the precipitate, wash the precipitate with distilled water, and dry to obtain dark green chlorophyllin solid;
[0030] (3) Weigh 1 g of the chlorophyllin solid in step (2), add 40 mL of methanol and an appropriate amount of sulfuric acid catalyst, heat to 40 ° C for 2 h, after the reaction, remove the methanol by rotary evaporation, and the resulting crude product...
specific Embodiment 3
[0031] Specific Example 3: Synthesis of Chlorophyllinamide Derivatives
[0032] (1) Weigh 1g of silkworm excrement chlorophyll primary extract, add 10mL of acetone to dissolve, stir for 30min, add 20ml of concentrated NaOH solution, place in a constant temperature water bath at 50-60°C and stir for 2h, after it is fully hydrolyzed, let it stand for 2h;
[0033] (2) Suction filter the precipitate obtained from the above step, dissolve it in 30mL water, and extract three times with 30mL petroleum ether; then adjust the pH of the solution to about 1 with 18% HCl, leave it at room temperature for 2 hours, and there is precipitation. The precipitate was collected by filtration, washed with distilled water, and dried to obtain a dark green chlorophyllin solid;
[0034] (3) Weigh 0.5 g of the chlorophyllin solid in step (2), add 30 mL of ammonia water, and heat to 60° C. for 2 hours. After the reaction, the ammonia water is removed by rotary evaporation, and the obtained crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com