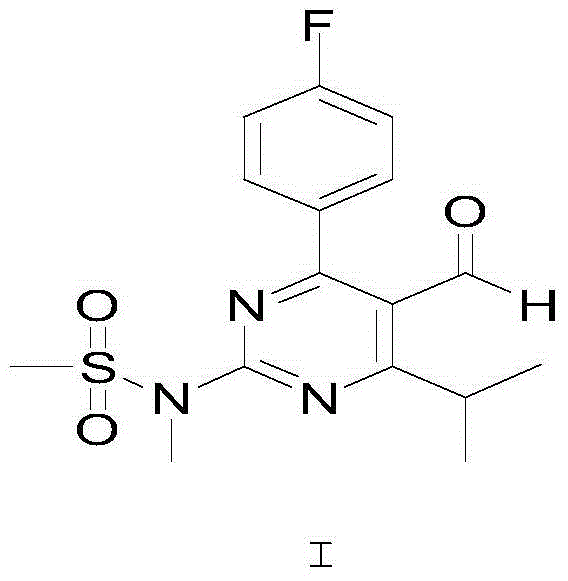
Synthetic method of rosuvastatin calcium key intermediate
A technology for the synthesis of rosuvastatin calcium and its synthesis method, which is applied in the field of synthesis of key intermediates of rosuvastatin calcium, can solve the problems that do not conform to the development concept of green chemistry, are not conducive to the health of operators, and are not conducive to large-scale production. Achieve the effects of easy large-scale production, environmental protection, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
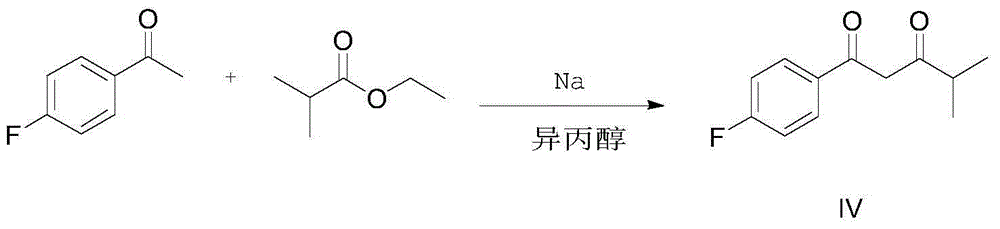
[0034] Example 1: Synthesis of 1-(4-fluorophenyl)-4-methylpentane-1,3-dione (compound IV)
[0035]
[0036] Add 150g of isopropanol to a 0.5L three-neck flask, then add 6.9g of sodium in batches, stir vigorously after the sodium is completely dissolved, add 13.81g (0.1mol) of p-fluoroacetophenone and 11.62g of ethyl isobutyrate dropwise (0.1mol) in 80g of isopropanol. The reaction solution was refluxed at 82° C. for 6 h, then cooled to room temperature, and stirred overnight. A large amount of product was precipitated, filtered to obtain an off-white product, and dried in a blast oven at 40°C to constant weight to obtain 18g, with a yield of 86%. NMR data (1HNMR, 500MHz, internal standard TMS, solvent CDCl3) is as follows: 1.30 (d, J=7.0Hz, 6H, CH 3 ), 2.61(m, 1H, CH), 4.15(s, 1H, CH 2 ), 7.18-7.12 (m, 2H, Ar-H), 7.93-7.87 (m, 2H, Ar-H).
Embodiment 2
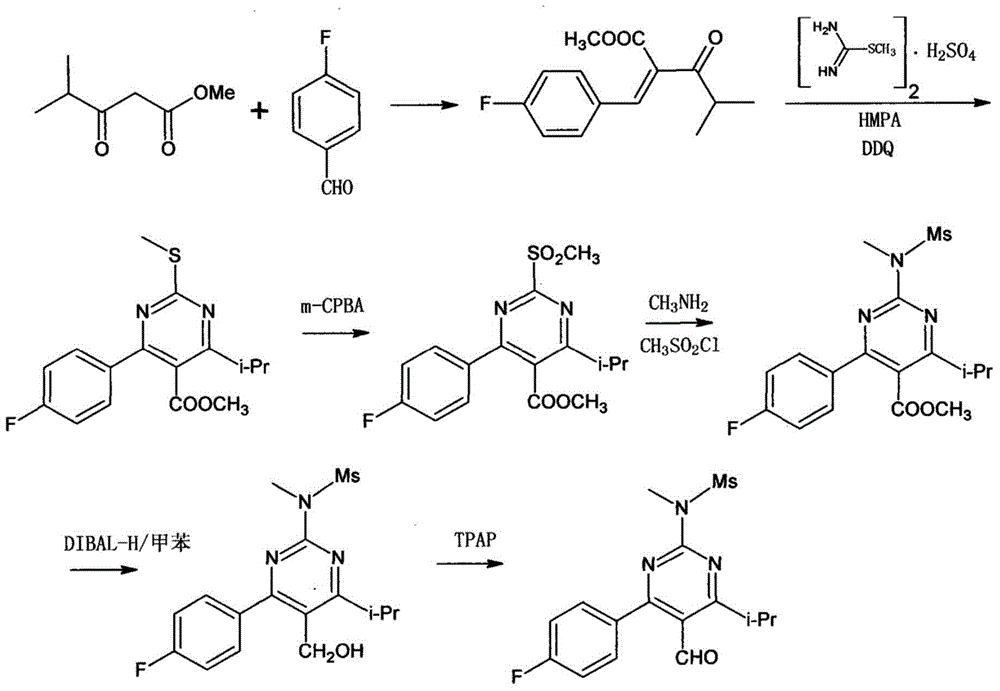
[0037] Example 2: Synthesis of 4-(4-fluorophenyl)-6-isopropyl-N-methylpyrimidin-2-amine (compound III)
[0038]
[0039] Add 10.4g (0.05mol) of compound IV, 6g (0.055mol) of methylguanidine hydrochloride, 8.4g (0.15mol) of potassium hydroxide, and 100ml of isopropanol into a 250ml three-necked flask, and heat up to reflux overnight. After the reaction was completed, the isopropanol was distilled off under reduced pressure, naturally cooled to 10°C, filtered, and a small amount of isopropanol was used to rinse the filter cake, and the resulting filter cake was dried in a vacuum oven at 50°C to constant weight. 11.4 g of off-white solid was obtained with a yield of 93%. NMR data (1HNMR, 500MHz, internal standard TMS, solvent DMSO) are as follows: 1.25(d, J=6.8Hz, 6H, CH 3 ), 2.98 (d, 3H, CH 3 ), 3.18(m, 1H, CH), 4.98(s, 1H, NH), 6.71(s, 1H, Ar-H), 7.16~7.10(m, 2H, Ar-H), 7.52~7.47(m, 2H, Ar-H).
Embodiment 3
[0040] Example 3: Synthesis of 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidine (Compound II)
[0041]
[0042]Under nitrogen protection, 9.8 g (0.04 mol) of compound III and 100 ml of dichloromethane were added to a 250 ml three-necked flask, and cooled to 5°C. , Add 12.1g (0.12mol) triethylamine, stir and react for half an hour. A solution of 5.5 g (0.048 mol) of methanesulfonyl chloride dissolved in 5 ml of dichloromethane was slowly added dropwise to the reaction solution, and the reaction was continued with stirring at 0-25° C. for 12 h. After the reaction was completed, 20ml of dichloromethane was added, 30ml of purification was added, concentrated hydrochloric acid was added to adjust the pH to 2-3, and the organic layer was obtained by separating the layers. The obtained organic layer was washed once with 50 ml of saturated brine, dried with 10 g of anhydrous sodium sulfate, and filtered. Dichloromethane was removed by desolvation unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com