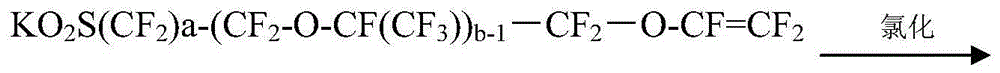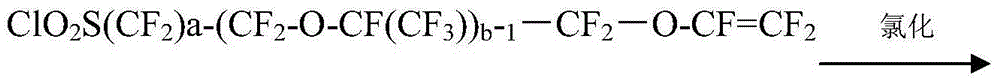Method for preparing perfluoro alkene ether sulfonyl fluoride compound
A technology of perfluoroalkenyl ether sulfonyl fluoride compound and perfluoroalkenyl ether sulfonyl chloride is applied in the preparation of sulfonic acid, organic chemistry and other directions, which can solve the problems of harsh catalyst preparation, large amount of chlorine gas and high temperature, and achieve strong proton exchange. The effect of the transfer function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] A method for preparing perfluoroalkenyl ether sulfonyl fluoride compound, the steps are as follows:
[0078] (1) Preparation of perfluoroiodoalkenyl ether fluoride
[0079] After boiling, washing and drying the 5L304 stainless steel high-pressure reactor equipped with a circulating cooling / heating system, temperature control system, feeding system, and stirring system, replace it with high-purity nitrogen several times until the oxygen content is below 10ppm and the moisture content is low. Below 250ppm, check that the airtightness is qualified and there is no leakage for 8 hours; evacuate to -0.1MPa, add 2.0L of diethylene glycol dimethyl ether through the feeding system, add 100g of cesium fluoride (cesium fluoride is baked at 350°C for 24 hours and ground ) and 1.12㎏ iodoacetyl fluoride, start to stir and circulate to cool down to -10°C, then start to feed hexafluoropropylene oxide, keep the pressure at -0.1MPa~0.1MPa for reaction, stop stirring after 5h of reaction,...
Embodiment 2
[0096] The method for preparing perfluoroalkene ether sulfonyl fluoride as described in Example 1, the difference is that the preparation process of step (1) perfluoroiodoalkene ether fluoride is as follows:
[0097] After boiling, washing and drying the 5L304 stainless steel high-pressure reactor equipped with a circulating cooling / heating system, temperature control system, feeding system, and stirring system, replace it with high-purity nitrogen several times until the oxygen content is below 10ppm and the moisture content is low. Below 250ppm, check that the airtightness is qualified and there is no leakage for 8 hours; evacuate to -0.1MPa, add 2.0L of diethylene glycol dimethyl ether through the feeding system, add 40g of potassium fluoride (potassium fluoride is baked at 350°C for 24 hours and ground ) and 1.07㎏ iodoacetyl fluoride, start to stir and circulate to cool down to -15°C, then start to feed hexafluoropropylene oxide, keep the pressure at -0.1MPa~0.1MPa for reac...
Embodiment 3
[0100] The method for preparing perfluoroalkene ether sulfonyl fluoride as described in Example 1, the difference is that the preparation process of step (1) perfluoroiodoalkene ether fluoride is as follows:
[0101] After boiling, washing and drying the 5L304 stainless steel high-pressure reactor equipped with a circulating cooling / heating system, temperature control system, feeding system, and stirring system, replace it with high-purity nitrogen several times until the oxygen content is below 10ppm and the moisture content is low. Below 250ppm, check that the airtightness is qualified, and there is no leakage for 8 hours; evacuate to -0.1MPa, add 2.0L of diethylene glycol dimethyl ether through the feeding system, add 38g of potassium fluoride (potassium fluoride is baked at 350°C for 24 hours and ground) ) and 1.3㎏ iodopropionyl fluoride, start stirring and circulate to cool down to -10°C, start to feed hexafluoropropylene oxide, keep the pressure at -0.1MPa~0.1MPa to react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com