N-acetylgutamate kinase mutant with heat stability improved remarkably
A technology of glutamic acid kinase and thermal stability, which is applied in the field of bioengineering and can solve the problems of poor thermal stability of NAGK
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Construction of embodiment 1 mutant expression plasmid and acquisition of recombinant E.coli BL21 strain
[0020] According to the argB gene sequence of Corynebacterium crenatum SYPA5-5, design the two primers of the N-acetylglutamate kinase coding gene, and then design the PCR point mutation intermediate primer according to the amino acid site to be mutated:
[0021] The mutant gene was amplified in vitro by overlap extension PCR.
[0022] The primers used for site-directed mutagenesis were:
[0023] PargB F: 5'-CGCGAATTCATGAATGACTTGATCAAAG-3' (EcoRI)
[0024] PargB R: 5'-CGCGTCGACTTACAGTTCCCCATCCTTG-3'(SalI)
[0025] Parg B K234T Fm: 5'-GTG TCCAAGATCA CTGCCACCGA GCTG-3'
[0026] Parg B K234T Rm: 5'-CAGCTCGGTG GCAGTGATCT TGGACAC-3'
[0027] Extracting the genome of Corynebacterium bacilli SYPA5-5 as a template;
[0028] PCR reaction conditions: pre-denaturation at 95°C for 5min, denaturation at 95°C for 30s, annealing at 58°C for 30s, extension at 72°C for 60s...
Embodiment 2
[0029] Retransform the recombinant plasmid with correct sequencing into E.coli BL21(DE3) competent cells to obtain recombinant E.coli BL21 strain Example 2 Expression of mutant N-acetylglutamate kinase and Ni-NTA purification
[0030] Inoculate the recombinants stored in cryopreserved tubes into LB medium containing kanamycin (final concentration: 50 μg / mL), culture overnight at 37°C with shaking, transfer at 1% inoculum size, and culture at 37°C until the OD is about 0.6- 0.8, add human IPTG to a final concentration of 1 mmol / L, and induce expression overnight at 16°C. Centrifuge the overnight induced expression bacterial solution at 10000r / min, 4°C for 15min, collect the bacterial cells, suspend the bacterial cells with Tris-HCI (pH8.0) buffer solution, break the cells by ultrasonic, and then filter through a 0.45μm filter membrane, select the expression The vector pET-28a contains 6His-Tag, NAGK is purified by Ni-NTA, and the pure NAGK enzyme protein is analyzed by SDS-PAGE...
Embodiment 3
[0031] The enzyme activity assay of embodiment 3 mutants
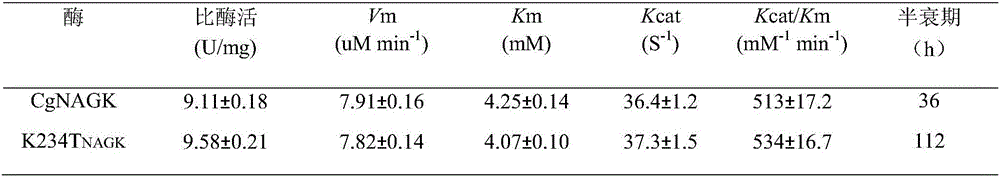
[0032] Carry out the enzymatic reaction of the pure enzyme solution obtained in the previous step: the total volume of the enzymatic reaction is 3mL, containing 595mmol / L Tris-HCl (pH8.0), 20mmol / L N-acetylglutamic acid, 20mmol / L MgCl 2 , 20mmol / L ATP disodium salt, 149mmol / LNH 2 0H . HCI and an appropriate amount of crude enzyme solution; after reacting at 37°C for 1 hour, add 1 mL of reaction termination solution (1.0mol / L HCI containing 5% FeCl 3 . 6H 2 0,4% trichloroacetic acid) to terminate the reaction; centrifuge, take the supernatant to measure the absorbance value A of N-acetylglutamic acid hydroxamic acid540 . The values of Km, Vm and Kat were measured by changing the concentration of the substrate-N-acetylglutamic acid (NAG) and using the double reciprocal method. The results obtained are shown in Table 1: N-acetylglutamate kinase mutant K234T NAGK The kinetic parameters of the wild type (CgNAGK) are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com