Zinc-sulfide, cuprous-sulfide and carbon nanocomposite and preparing method thereof
A technology of cuprous sulfide and composite materials, applied in the direction of zinc sulfide, etc., can solve the problems of increased cost, environmental pollution, not as economical as in-situ synthesis, etc., to enhance stability, improve catalytic efficiency, and promote the separation of electrons and holes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Prepare a mixed salt solution of zinc nitrate and copper nitrate, in which Zn 2+ The concentration is 0.08M, Cu 2+ The concentration is 0.02M; prepare a sodium benzoate solution with a concentration of 0.03M; add the above two solutions into the four-necked bottle at the same time, the molar ratio of sodium benzoate to zinc nitrate is 2, take 0.5M sodium hydroxide solution to adjust the pH value 6.2, keep the reaction temperature at 80°C, after crystallization for 24 hours, wash and dry at low temperature; store the prepared precursor at room temperature under H 2 React in S atmosphere for 1 minute, H 2 S gas flow rate is 100mL min -1 , to obtain zinc sulfide, copper sulfide and benzoic acid nanocomposite materials, and then roast the zinc sulfide, copper sulfide and benzoic acid composite nanomaterials at 650°C for 8 hours in a nitrogen atmosphere to obtain zinc sulfide, cuprous sulfide and carbon nanocomposites.
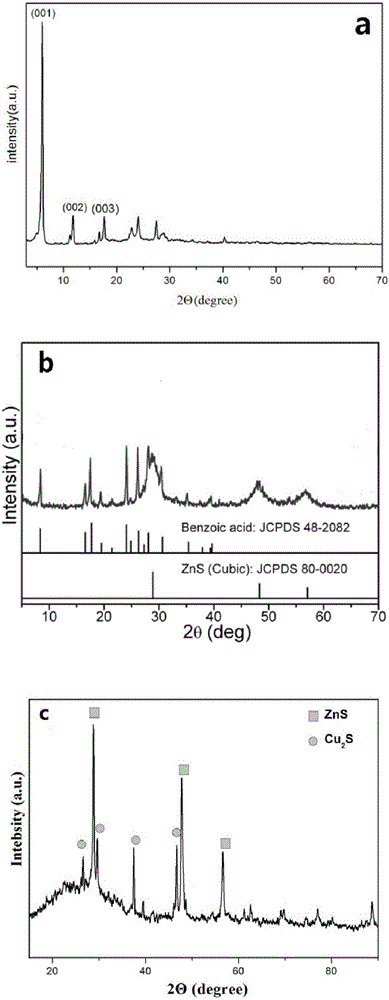
[0025] from figure 1 -a It can be seen that the be...
Embodiment 2
[0030]Prepare a mixed salt solution of zinc nitrate and copper nitrate, wherein the concentration of Zn2+ is 0.05M, and the concentration of Cu2+ is 0.05M; the sodium benzoate solution with a concentration of 0.05M is prepared; the above two solutions are added to the four-necked bottle simultaneously, and salicylic acid The molar ratio of sodium to zinc nitrate is 0.5. Add the above two solutions into the four-neck flask at the same time, take 0.5M sodium hydroxide solution to adjust the pH value to 7, keep the reaction temperature at 80°C, crystallize for 24 hours, wash, Dry at low temperature, react the prepared precursor in H2S atmosphere at room temperature for 60 minutes to obtain composite nanomaterials of zinc sulfide, copper sulfide and benzoic acid, and then place the composite nanomaterials of zinc sulfide, copper sulfide and benzoic acid at 650°C in nitrogen Calcined under atmosphere for 8h to obtain zinc sulfide, cuprous sulfide and carbon nanocomposites.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com