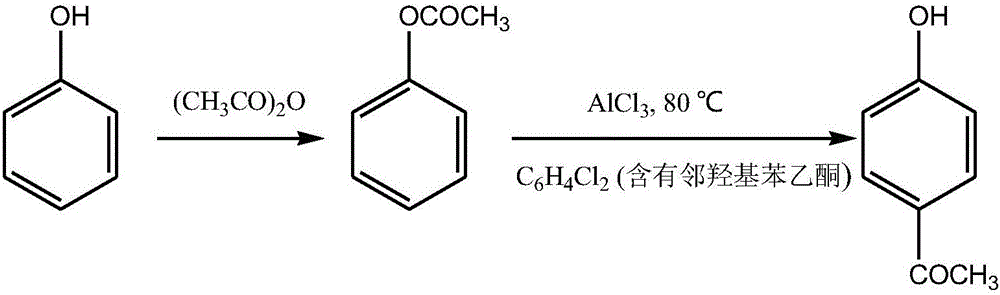
Synthetic method of p-hydroxyacetophenone
A technology of p-hydroxyacetophenone and o-hydroxyacetophenone is applied in the field of synthesis of p-hydroxyacetophenone, and can solve the problems of low yield of p-hydroxyacetophenone, unsatisfactory, unsuitable for industrial production and the like, and achieves The effect of improving yield, increasing content, and inhibiting formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of synthetic method of p-hydroxyacetophenone, carries out according to the following steps:
[0028] (1) Esterification reaction: In a 500ml reaction bottle, put 62.2g (0.66mol) of phenol and 70.8g (0.69mol) of acetic anhydride, stir and cool to 5°C, add the first drop of concentrated sulfuric acid dropwise, and wait for the reaction to drop to Then add the second drop of concentrated sulfuric acid dropwise at room temperature. The dosage of the two drops of concentrated sulfuric acid is 0.5g; Pour the reaction solution into 2 times the amount of water, adjust the pH to 8-9 with alkali 5% sodium hydroxide, extract 3 times with ethyl acetate, combine the extracts, dry with anhydrous magnesium sulfate, filter, and concentrate to obtain phenyl acetate Liquid 84.6g, yield 95%; divided into three spare;
[0029] (2) Fries rearrangement: Take 27.2g (0.2mol) of phenyl acetate obtained in step (1), put it in a 250ml reaction bottle, put 30ml of o-dichlorobenzene under s...
Embodiment 2
[0035] A kind of synthetic method of p-hydroxyacetophenone, carries out according to the following steps:
[0036] (1) Esterification reaction: Put 60g (0.63mol) of phenol and 70.0g (0.68mol) of acetic anhydride into a 500ml reaction bottle, stir and cool to 10°C, add the first drop of concentrated sulfuric acid dropwise, and wait for the reaction to cool to room temperature Then add the second drop of concentrated sulfuric acid dropwise. The amount of concentrated sulfuric acid added dropwise is 0.5g; Pour the solution into 3 times the amount of water, adjust the pH to 8-9 with 5% sodium hydroxide, extract 3 times with ethyl acetate, combine the extracts, dry with anhydrous magnesium sulfate, filter, and concentrate to obtain phenyl acetate liquid 84.3 g, yield 94.6%; be divided into three standby;
[0037] (2) Fries rearrangement: Take 27.2g (0.2mol) of phenyl acetate obtained in step (1), put it in a 250ml reaction bottle, put 30ml of o-dichlorobenzene under stirring condi...
Embodiment 3
[0043] A kind of synthetic method of p-hydroxyacetophenone, carries out according to the following steps:
[0044] (1) Esterification reaction: In a 500ml reaction bottle, put 62.5g (0.66mol) of phenol and 71.5g (0.70mol) of acetic anhydride, stir and cool to 5°C, add the first drop of concentrated sulfuric acid dropwise, and wait until the reaction drops to Then add the second drop of concentrated sulfuric acid dropwise at room temperature. The dosage of the two drops of concentrated sulfuric acid is 0.5g; Pour the reaction solution into 2 times the amount of water, adjust the pH to 8-9 with 5% sodium hydroxide, extract 4 times with ethyl acetate, combine the extracts, dry with anhydrous magnesium sulfate, filter, and concentrate to obtain phenyl acetate liquid 84.9g, yield 95.3%; divided into three spare;
[0045] (2) Fries rearrangement: Take 27.2g (0.2mol) of phenyl acetate obtained in step (1), put it in a 250ml reaction bottle, put 30ml of o-dichlorobenzene under stirri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com