Metabolism marker related to idiopathic male infertility in urine, and detection method and application thereof
A technology for metabolic markers and male infertility, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems of prolonging the experimental cycle, long detection time, and limiting applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1: Research object selection and group basis
[0106] The subjects of this part of the study are from the first-diagnosed idiopathic male infertility cases and healthy childbearing controls in the Affiliated Hospital of Nanjing Medical University. The research content and the informed consent form were approved by the Ethics Committee of Nanjing Medical University and complied with the requirements of relevant laws and regulations. Cases and controls signed the informed consent after understanding the content. All subjects underwent a complete physical examination and completed a questionnaire including basic personal information, living habits, occupational and environmental exposures, genetic risk factors, sexual and reproductive function, disease history, and physical activity. The first stage included 607 idiopathic male infertility cases and 430 healthy controls who met the requirements; the second stage included 15 idiopathic male infertility cases and...
Embodiment 2
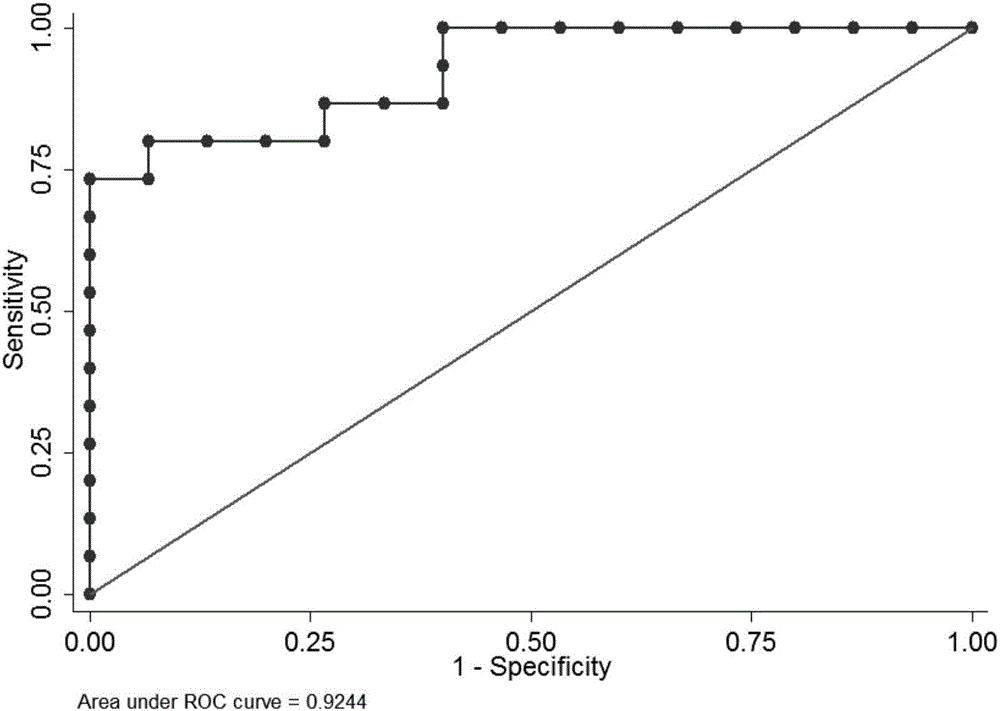
[0137] Example 2: UPLC-MS metabolomics biomarker screening for idiopathic male infertility
[0138] 1. Sample pretreatment
[0139] 1.1. Take 300 μL of urine, add 10 μL of internal standard A, add 10 μL of internal standard B, add 10 μL of internal standard C, add 40 μL of methanol (reagent A), and vortex for 30 seconds.
[0140] 1.2. Centrifuge at 16000g at 4°C for 15min in a centrifuge, transfer the supernatant to a 1.5mL imported EP tube, and concentrate the supernatant to dryness in a centrifugal concentrator at room temperature.
[0141] 1.3. Reconstitute with 5 μL ultrapure water (reagent D) and wait for analysis.
[0142] 2. Instrument testing
[0143] 2.1. Analytical instruments: UPLC Ultimate 3000system (Dionex) high-performance liquid chromatography; Q-Exactive high-resolution mass spectrometer.
[0144] 2.2. Liquid phase conditions:
[0145] 2.2.1 The liquid chromatographic column is a Hypersil GOLD C18 chromatographic column (100mm×2.1mm, particle size 1.9μm, T...
Embodiment 3
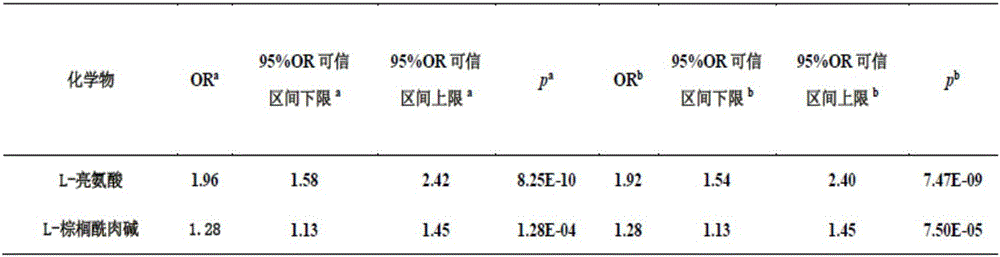
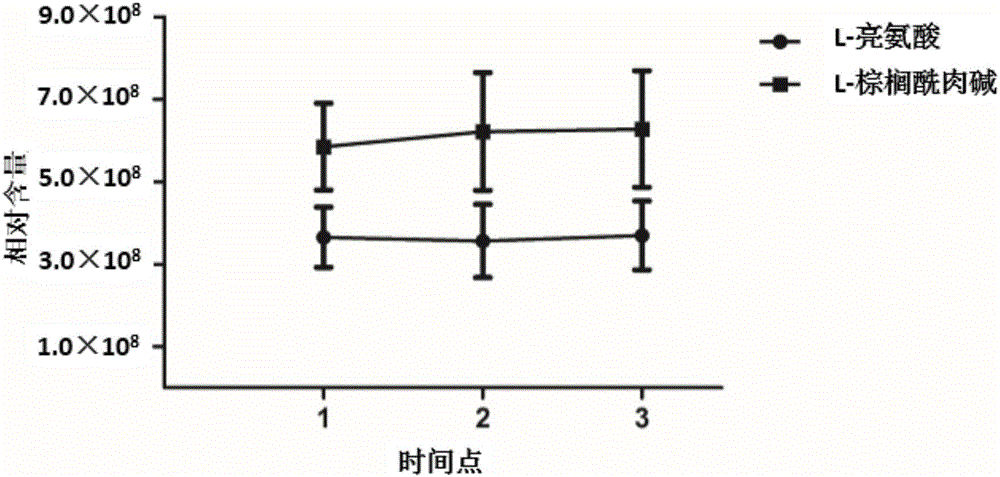
[0158] Example 3: Stability analysis of L-leucine and L-palmitoylcarnitine in urine
[0159] The stability of the levels of L-leucine and L-palmitoylcarnitine in urine was evaluated by the method of Example 2 (the interval was 2 weeks). The results showed that the levels of L-leucine and L-palmitoylcarnitine in urine were stable ( figure 2 ), with properties as diagnostic / monitoring markers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com