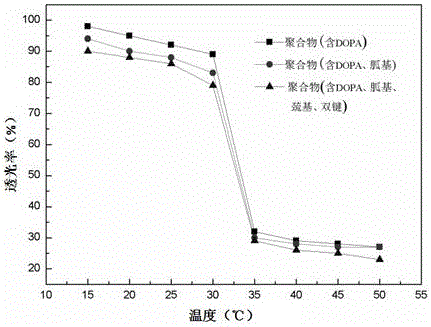
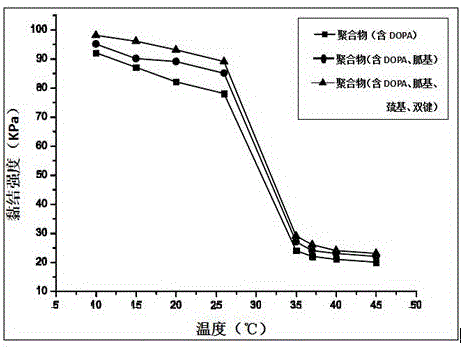
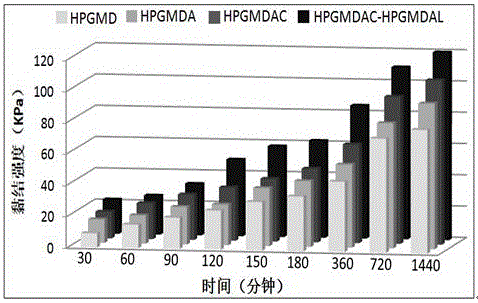
Temperature response type polymeric biomedical adhesive and synthesis method thereof
A biomedical, temperature-responsive technology, applied in the field of polymer materials and biomedical materials, can solve the problems of slow curing, body fluid leakage, insufficient strength, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: HP (DOPA-Glu-EG 2 ) preparation
[0035] Glu-EG 2 Preparation: Add 2.5 g (16.99 mmol) of glutamic acid and 15 mL (129.21 mmol) of diethylene glycol mono Methyl ether, 2 mL of concentrated sulfuric acid was added to a constant pressure dropping funnel, and slowly added to a three-necked flask under nitrogen protection at 0 °C and reacted for 12 h under ice bath to obtain a crude product. Pour the crude product into a mixture of 50 mL of triethylamine and 50 mL of ethanol to obtain a white precipitate, centrifuge, dissolve the white precipitate in 10 mL of methanol, then pour the solution into the white precipitate in 100 mL of ether, and centrifuge to obtain The white precipitated product was vacuum-dried to obtain a white solid that was L-glutamic acid-β-diethylene glycol monomethyl ether ester (Glu-EG 2 ), the yield was 55%.
[0036] Preparation of Glu-EG2-NCA: Add 1 g (4.02 mmol) Glu-EG to a 100 mL three-necked flask equipped with a magnetic stir...
Embodiment 2
[0040] Embodiment 2: HP (Arg-DOPA-Glu-EG 2 )preparation
[0041] Glu-EG 2 The preparation: with embodiment 1;
[0042] Glu-EG 2 -Preparation of NCA: with embodiment 1;
[0043] Preparation of DOPA-NCA: same as Example 1;
[0044] HP (DOPA-Glu-EG 2 ) preparation: with embodiment 1;
[0045] Preparation of Arg-NCA: Add 6.6 g (21.43 mmol) of benzyloxycarbonyl arginine and 30 mL of solvent tetrahydrofuran into a 100 mL three-necked flask equipped with a magnetic stirrer, thermometer, constant pressure dropping funnel and nitrogen protection device. Add 5 mL of tetrahydrofuran solution containing 1 mL of phosphorus tribromide (10.64 mmol) into the constant pressure dropping funnel. Under nitrogen protection, it was added dropwise into a three-necked flask under ice-cooling, and then reacted at room temperature for 16 hours. After the reaction, the reaction solution was separated into layers, and the tetrahydrofuran solution in the upper layer was poured out, and t...
Embodiment 3
[0048] Embodiment 3: HP (Cys-Arg-DOPA-Glu-EG 2 ) preparation
[0049] Glu-EG 2 The preparation: with embodiment 1;
[0050] Glu-EG 2 -Preparation of NCA: with embodiment 1;
[0051] Preparation of DOPA-NCA: same as Example 1;
[0052] HP (DOPA-Glu-EG 2 ) preparation: with embodiment 1;
[0053] Preparation of Arg-NCA: same as Example 2;
[0054] HP(Arg-DOPA-Glu-EG 2 ) preparation: with embodiment 2;
[0055] Preparation of Cys-NCA: Add 1 g (8.25 mmol) cysteine and 30 mL of anhydrous-treated tetrahydrofuran into a 100 mL three-necked flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel and a nitrogen protection device, Add 10 mL of tetrahydrofuran solution with 1.47 g (4.96 mmol) of triphosgene dissolved in the constant pressure dropping funnel. Raise the temperature to about 50 °C, and slowly add the triphosgene solution in the constant pressure dropping funnel into the three-necked flask under magnetic stirring. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com