Immobilization method of sucrose isomerase
A sucrose isomerase, sucrose technology, applied in the directions of isomerase, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of complex methods, high cost of carriers, and difficulty in large-scale industrial application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 B.brevis / pNY 326-pal I LSP build
[0035] At the 5' and 3' ends of the sucrose isomerase gene (pal I) (see SEQ ID NO.1 for the gene sequence), the restriction sites Nde I and Hind III were designed and introduced, and finally provided by Shanghai Jierui Bioengineering Co., Ltd. Synthesized to obtain pUC57-palI.
[0036] The plasmid pUC57-palI carrying pal I and the expression vector pET-24a(+) were digested with restriction endonucleases NdeI and Hind III respectively, and the target fragment was recovered and ligated to obtain the recombinant plasmid pET-24a-palI.
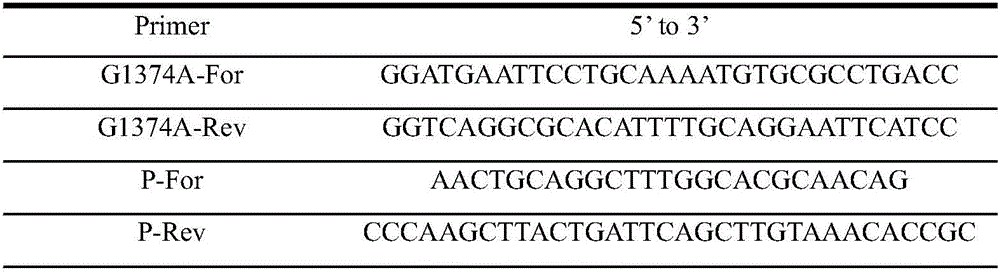
[0037]Using pET-24a(+)-pal I as a template, design the PCR primers shown in Table 1G1374A-For and G1374A-Rev, and use one-step PCR to perform site-directed mutation at the G1347 site of the sucrose isomerase gene, and remove the inner part of pal I by mutation The pst I site; then use primers P-For, P-Rev PCR amplification to obtain the target gene fragment palI with Pst I and Hind III restricti...
Embodiment 2
[0041] The impact of embodiment 2 shake flask culture medium on fermented liquid enzyme activity
[0042] Bacillus pumilus B.brevis / pNY 326-pal I LSP As the starting strain, after cultured in the seed medium, it was inserted into different shake flask medium:
[0043] (1) Single nitrogen source
[0044] With glucose as the carbon source, industrial yeast powder, industrial peptone, soybean peptone, beef extract, casein, cottonseed powder cake, poly-peptone, tryptone, beef peptone, angel peptone, etc. were used as nitrogen on TM medium. source, the content of each nitrogen source is 15g L -1 , the inoculum size was 1%, and the enzyme activity was determined after culturing on a shaker at 200r / min and 30°C for 48h.
[0045] (2) Compound nitrogen source
[0046] On the basis of a single nitrogen source, two better nitrogen sources were selected, mixed and prepared according to a certain ratio, the inoculum amount was 1%, and the enzyme activity was measured after culturing on...
Embodiment 3
[0060] Embodiment 3 Preparation of immobilized sucrose isomerase
[0061] (1) Bacillus pumilus (B.brevis / pNY 326-palI LSP ) is the starting strain, and the fermentation medium finally determined in Example 2 is used to ferment and prepare the sucrose isomerase enzyme liquid. After the fermentation is completed, the bacteria are removed by centrifugation to obtain the enzyme liquid.
[0062] (2) Using 4% acetic acid solution as a solvent, prepare a chitosan colloid solution with a final concentration of 2%-5%, and vacuumize to remove air bubbles.
[0063] (3) Slowly drop the fully dissolved chitosan colloid solution into 4M sodium hydroxide solution with a syringe, stir at a constant speed, and form chitosan microspheres with a diameter of about 1.5 mm. After the microspheres are completely formed, pour off the hydroxide Sodium solution, the prepared microspheres were washed with deionized water until neutral.
[0064] (4) Add the chitosan microspheres that have been washed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com