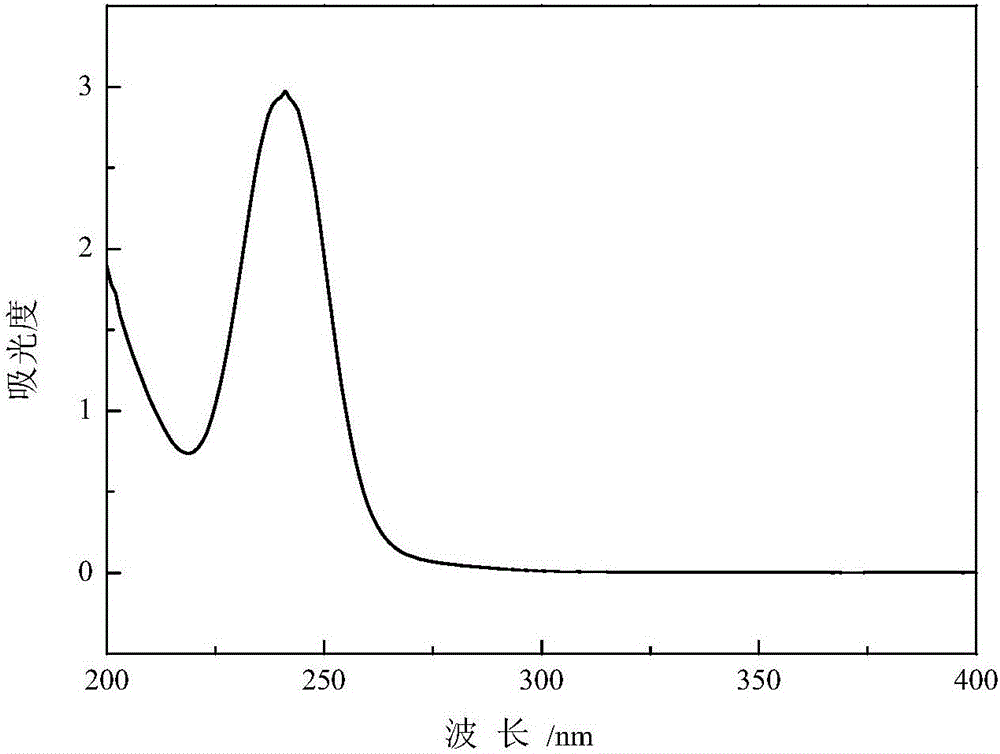
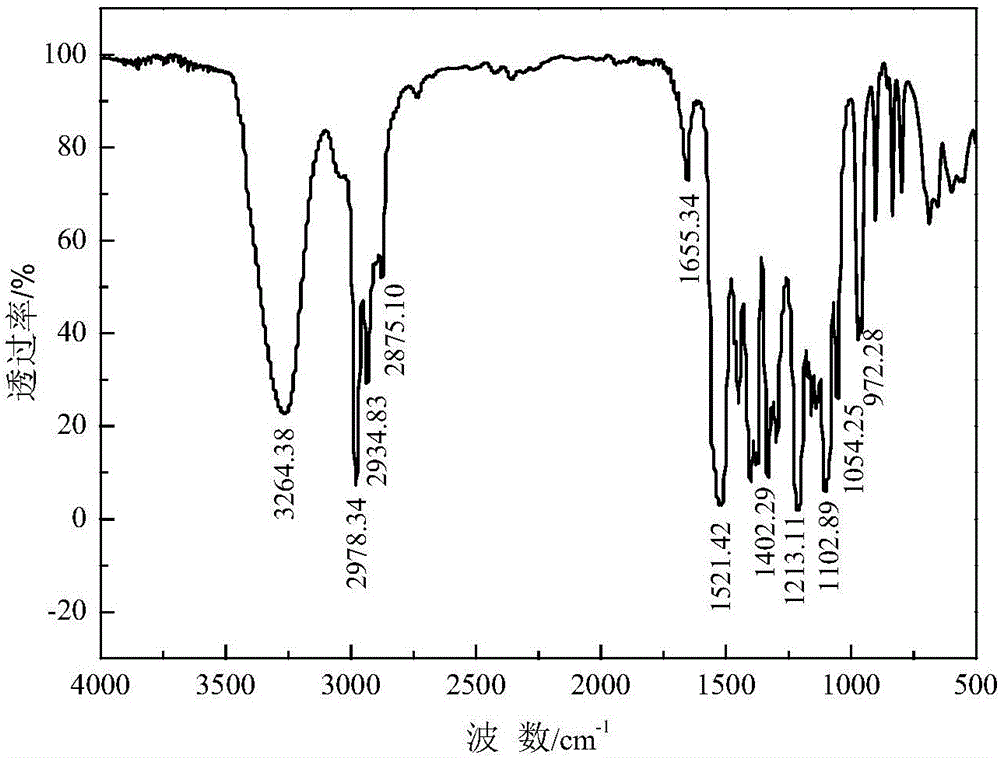
Method for producing thionocarbamate and dibenzyl disulfide
A technology of dibenzyl disulfide and thiocarbamate, which is applied in the field of preparing thiocarbamate and co-producing dibenzyl disulfide, producing thiocarbamate and dibenzyl disulfide, and can solve problems such as environmental pollution , to achieve high reactivity, increase yield, and avoid waste water and waste gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of O-isopropyl-N-ethylthiocarbamate and co-production of dibenzyl disulfide:
[0059] 15.16 parts of O-isopropyl-N-ethyl thiocarbamate with a purity of 95.12% and 8.77 parts of sodium isopropyl xanthate with a purity of 90.27% were added to the reactor, fully stirred, and then Add 6.40 parts of benzyl chloride with a purity of 99.0% to the pressure dropping funnel, control the reaction temperature to 75°C, cool to room temperature after 3 hours of reaction, filter to remove sodium chloride, transfer the filtrate to the reactor, and then use a constant pressure dropping funnel to Add 3.34 parts of ethylamine aqueous solution (content: 65% to 70%), heat up to 70°C, react for 2 hours, cool to room temperature, add 0.19 parts of tetra-n-butylammonium iodide (purity: 99.0%) to the reactor, And add 89.27 parts of hydrogen peroxide with a concentration of 2.0% with a constant pressure dropping funnel, stir and react at room temperature for 20 minutes, and the reactio...
Embodiment 2
[0061] Synthesis of O-isopropyl-N-ethylthiocarbamate and co-production of dibenzyl disulfide:
[0062] 30.32 parts of purity of 95.12% O-isopropyl-N-ethyl thiocarbamate and 17.54 parts of purity of 90.27% sodium isopropyl xanthate were added to the reactor, fully stirred, and then Add 12.80 parts of benzyl chloride with a purity of 99.0% to the pressure dropping funnel, control the reaction temperature to 75°C, react for 3 hours and cool to room temperature, filter to remove sodium chloride, transfer the filtrate to the reactor, and then use a constant pressure dropping funnel to Add 6.68 parts of ethylamine aqueous solution (content: 65% to 70%), heat up to 70°C, react for 2 hours, cool to room temperature, add 0.25 parts of tetra-n-butylammonium iodide (purity: 99.0%) into the reactor, Get 11.30 parts of hydrogen peroxide with a concentration of 30%, add 87.11 parts of the separated water phase in Example 1, and add 60.26 parts of distilled water, and add it dropwise to the ...
Embodiment 3
[0064] Synthesis of O-isobutyl-N-ethylthiocarbamate and co-production of dibenzyl disulfide:
[0065] 20.00 parts of O-isobutyl-N-ethylthiocarbamate with a purity of 94.77% and 9.42 parts of sodium isobutyl xanthate with a purity of 91.41% were added to the reactor, fully stirred, and then Add 6.40 parts of benzyl chloride with a purity of 99.0% to the pressure dropping funnel, control the reaction temperature to 65°C, react for 2 hours, cool to room temperature, filter to remove sodium chloride, transfer the filtrate to the reactor, and then use a constant pressure dropping funnel to Add 3.35 parts of ethylamine aqueous solution (content: 65% to 70%), heat up to 70°C, react for 2 hours, cool to room temperature, add 0.48 parts of tetra-n-butylammonium bromide (purity: 99.0%) to the reactor, And add 35.71 parts of 5.0% hydrogen peroxide with a constant pressure dropping funnel, stir and react at room temperature for 20 minutes, and the reaction ends. Stand at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com