An annonacein nanoparticle based on cyclodextrin and lecithin as carrier and its preparation method and application
A technology of cyclodextrin and nanoparticles, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problem of difficulty in maximizing the curative effect, low bioavailability, and limited in vivo research. Limitation and other issues, to achieve significant anti-tumor efficacy, no toxic side effects, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
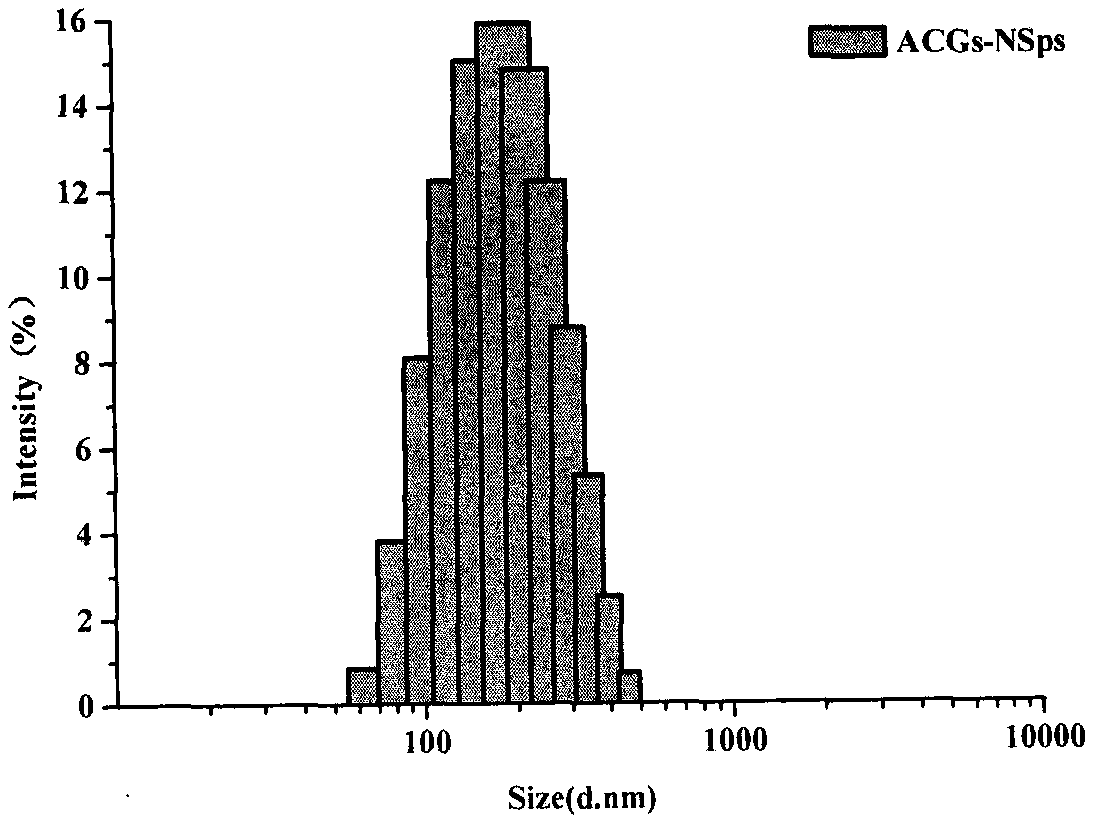
[0048]Weigh 2 mg of soybean lecithin and dissolve it in 0.2 mL of methanol, slowly inject it into 4 mL of aqueous solution containing 4 mg of hydroxypropyl-β-cyclodextrin at 25°C and 500 rpm under stirring conditions, continue stirring for 10 minutes, and then rotary evaporate to remove methanol. Subsequently, 8 mg of ACGs was dissolved in 0.4 mL of methanol, injected into the above-obtained solution at room temperature and stirred at 500 rpm, and then the methanol was removed by rotary evaporation to obtain ACGs nanoparticles. The average particle size is 144.4nm ( figure 1 ), the polydispersity index (PDI) was 0.08, and the potential value was -22.9mV.
Embodiment 2
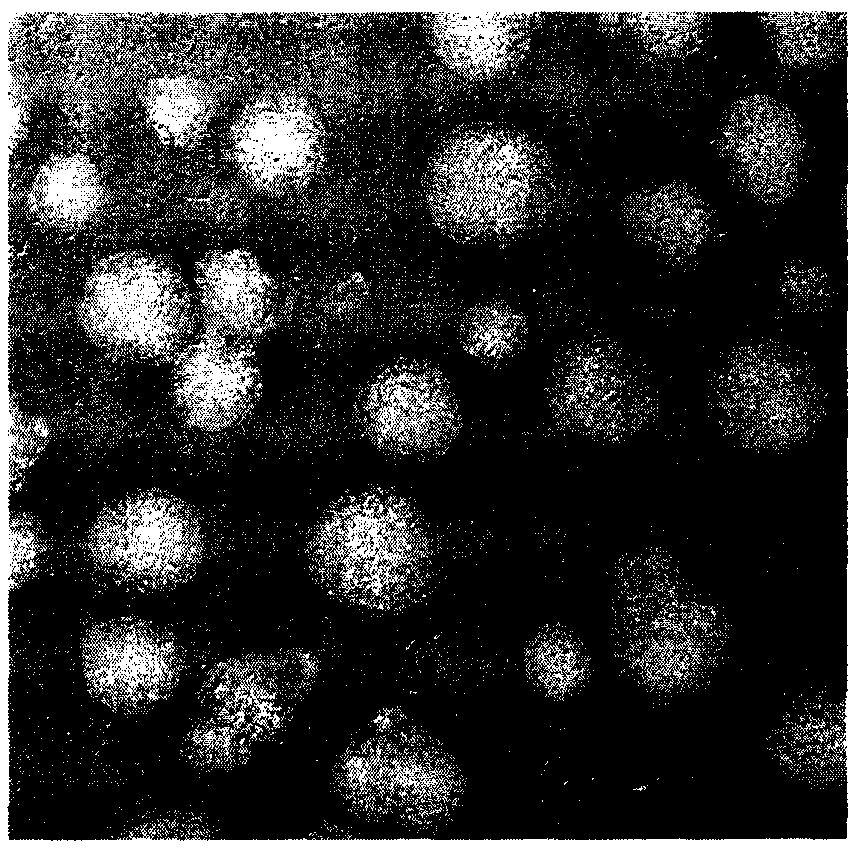
[0050] Prepare ACGs suspension at a concentration of 2 mg / mL, absorb 6 μL and drop it on a 300-mesh copper grid, let it dry naturally in the air, and then stain it with 0.1% uranyl acetate for 10 min, observe the morphology of the particles under a transmission electron microscope ( figure 2 ).
Embodiment 3
[0051] Example 3 Investigation of the Stability of ACGs Nanoparticles in Artificial Gastrointestinal Fluid
[0052] Preparation of artificial gastric juice: Take 16.4mL of dilute hydrochloric acid with a concentration of 1mol / L, add 800mL of distilled water, 10g of pepsin, mix well, add water to dilute to 1000mL.
[0053] Preparation of artificial intestinal juice: 6.8g potassium dihydrogen phosphate, add 500mL water, adjust pH to 6.8 with 0.1mol / L sodium hydroxide, take another 10g trypsin, add water to dissolve, mix the two liquids and add water to dilute to 1000mL.
[0054] Take 0.5mL of the prepared artificial gastrointestinal fluid after passing through the membrane, mix it with ACGs nanoparticles in equal volume, and measure the change of its particle size at a certain time point.
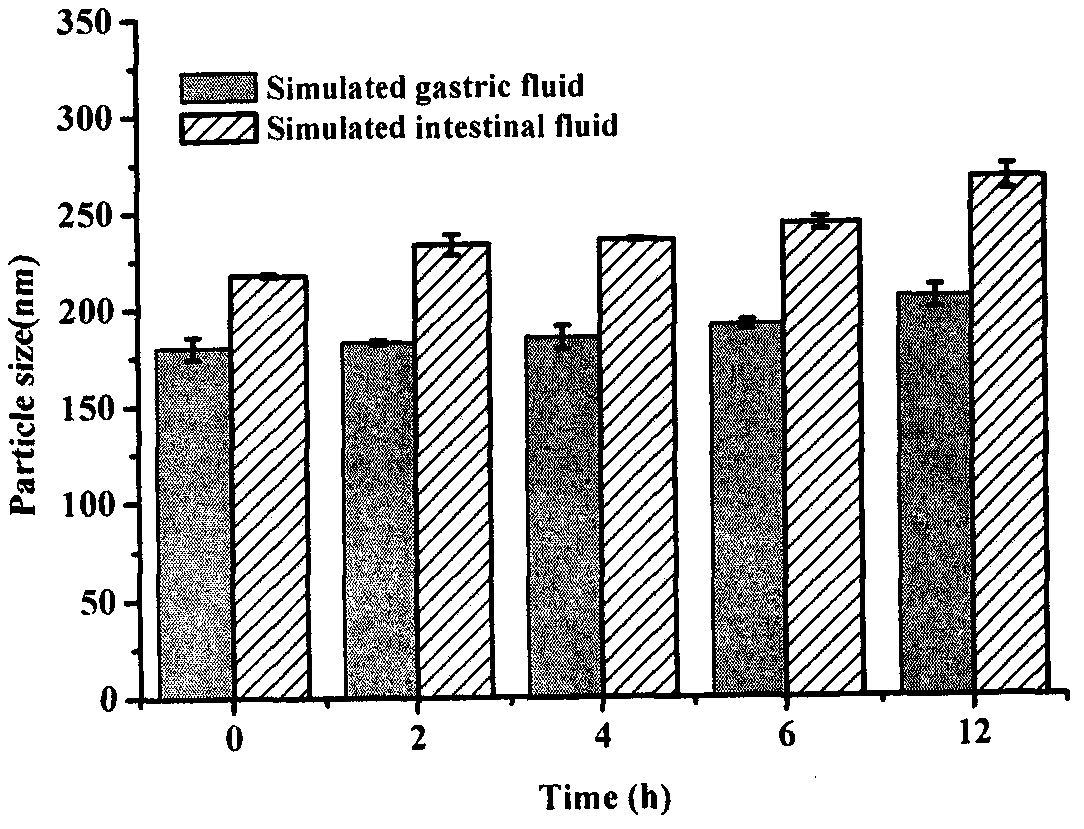
[0055] Results: In the artificial gastrointestinal fluid, the particle size of ACGs nanoparticles changed little within 12 hours ( image 3 ), indicating that ACGs nanoparticles are basicall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com