Tagatose preparation method
A technology of tagatose and phosphate tagatose, applied in the direction of fermentation, etc., can solve the problems of not being widely used, affecting the final price of tagatose, not suitable for large-scale production, etc., and achieve the effect of high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0060] Experimental example 1 in vitro multi-enzyme catalyzed conversion of starch into tagatose
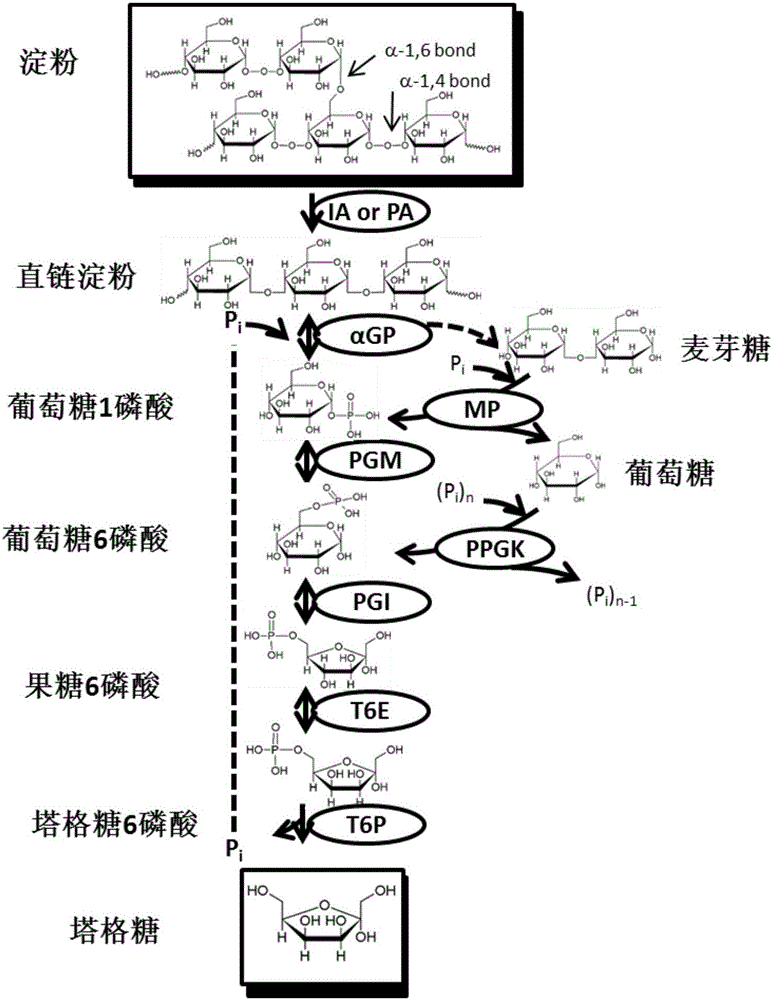
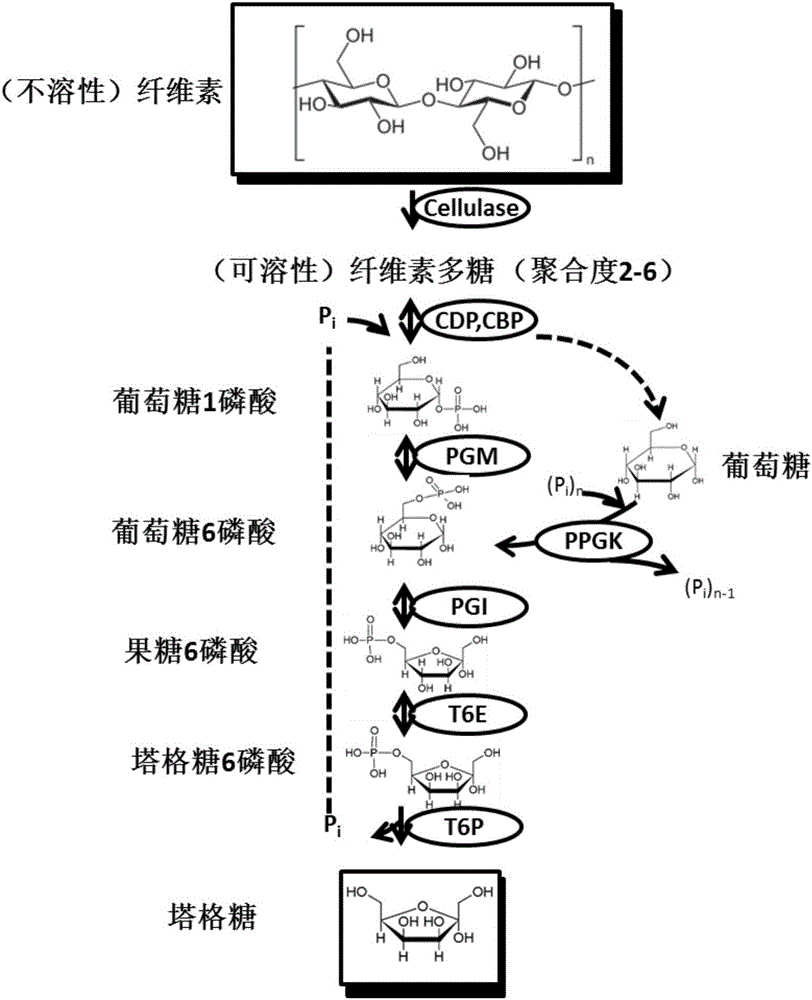
[0061] Starch was converted into tagatose by an in vitro multi-enzyme catalytic system ( figure 1 ). These key enzymes include: (1) α-glucan phosphorylase (αGP, EC 2.4.1.1), which releases 1-phosphate glucose from the non-reducing end of starch by adding 1 phosphate; Bitase (PGM, EC 5.4.2.2), which catalyzes glucose 1-phosphate to glucose 6-phosphate; (3) glucose phosphate isomerase, which converts glucose 6-phosphate to fructose 6-phosphate; (4) tower 6-phosphate Lattose epimerase, which converts fructose-6-phosphate to tagatose-6-phosphate; (5) 6-phosphate-tagatose phosphatase, which dephosphorylates tagatose-6-phosphate to tagatose and phosphoric acid.
[0062] In the present invention, α-glucan phosphorylase is derived from Thermotoga maritima, and the gene number on KEGG is TM1168; glucose phosphomutase is also derived from Thermotoga maritima, and the gene number on KEG...
experiment example 2
[0066] Experimental example 2 in vitro multi-enzyme catalyzed conversion of starch into tagatose
[0067] The preparation of α-glucan phosphorylase, glucose phosphomutase, glucose phosphate isomerase, 6-phosphate tagatose epimerase and 6-phosphate tagatose phosphatase was the same as in Experimental Example 1.
[0068] Contain 30mM phosphate buffer (pH 7.0) in a 0.75 milliliter reaction system, the divalent magnesium ion of 5mM, the consumption of described α-glucan phosphorylase is 10U / mL, described glucose phosphomutase The dosage is 10U / mL, the dosage of the glucose phosphate isomerase is 10U / mL, the dosage of the 6-phosphate tagatose epimerase is 10U / mL, the 6-phosphate tagatose phosphate The dosage of the enzyme is 10U / mL, 100g / L of soluble starch is used to catalyze the reaction at 20°C for 24 hours.
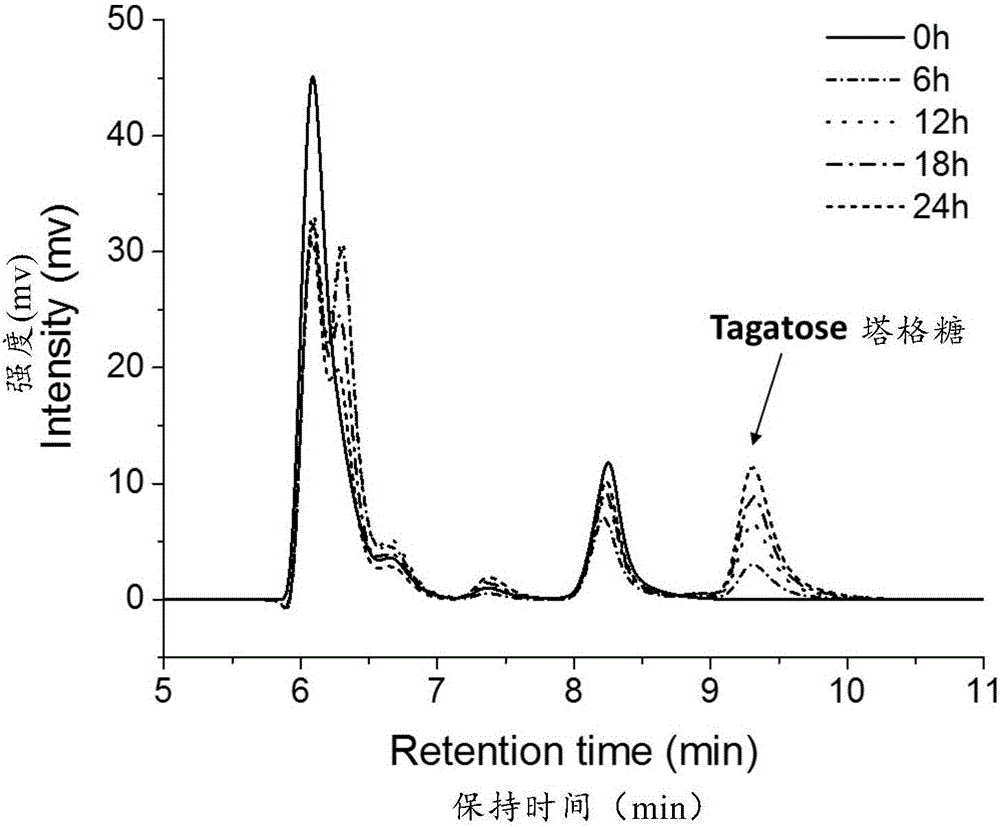
[0069] After the reaction, the final concentration of tagatose was 16g / L, and the conversion rate was 16%.
experiment example 3
[0070] Experimental example 3 in vitro multi-enzyme catalyzed conversion of starch into tagatose
[0071] The preparation of α-glucan phosphorylase, glucose phosphomutase, glucose phosphate isomerase, 6-phosphate tagatose epimerase and 6-phosphate tagatose phosphatase was the same as in Experimental Example 1.
[0072]Contain 30mM phosphate buffer (pH 7.0) in a 0.75 milliliter reaction system, the divalent magnesium ion of 5mM, the consumption of described α-glucan phosphorylase is 10U / mL, described glucose phosphomutase The dosage is 10U / mL, the dosage of the glucose phosphate isomerase is 10U / mL, the dosage of the 6-phosphate tagatose epimerase is 10U / mL, the 6-phosphate tagatose phosphate The amount of enzyme used is 10U / mL, 100g / L soluble starch, and the catalytic reaction is carried out at 50°C for 24 hours.
[0073] After the reaction, the final concentration of tagatose was 8g / L, and the conversion rate was 8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Final concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com