Intrinsic self-repairing recoverable polyurethane polymer, and preparation method and application thereof
A polyurethane polymer, self-healing technology, applied in the field of intelligent polymer materials, can solve the problems of the lack of dynamic reversibility of the DA bond, the inability to maintain the bearing capacity and shape of the material, and the large limitations, achieving easy synthesis and preparation, and simple structure. , the effect of short repair time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
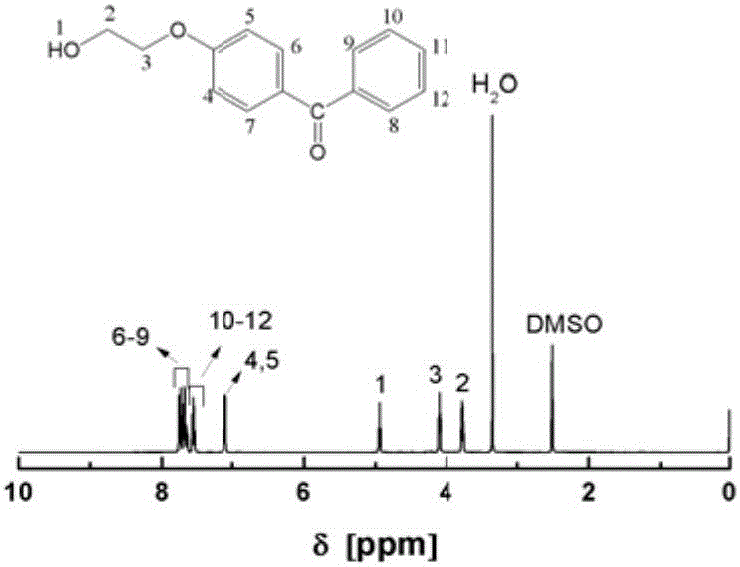
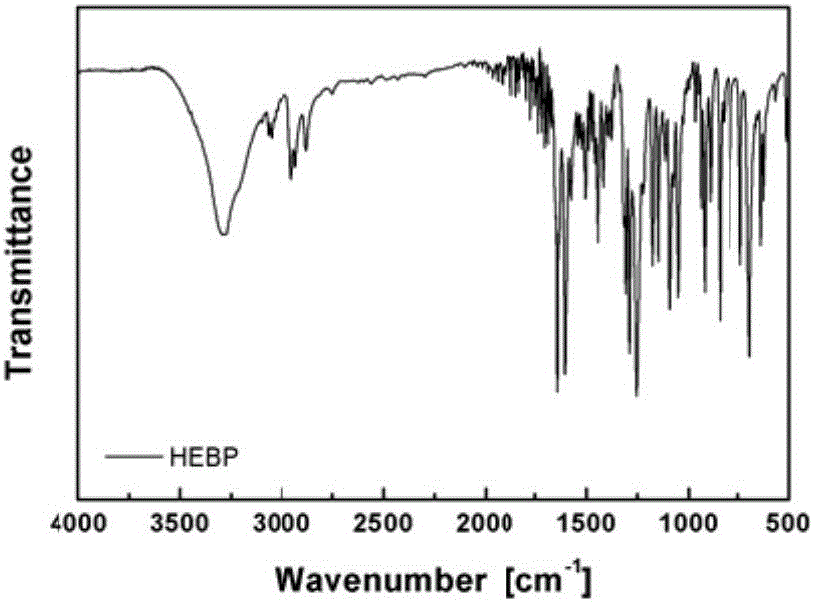
[0065] Add 8.0 parts of 4-hydroxybenzophenone into a 150ml three-neck flask with magnetic stirring, add 40 parts of DMF solvent and stir to dissolve, then add 7.4 parts of 2-bromoethanol dropwise at a uniform speed, and then add 12 parts of anhydrous Potassium carbonate was heated to 90°C for 18 hours under nitrogen atmosphere. After the reaction, the mixed solution was filtered to remove the precipitate, poured into distilled water and adjusted to pH = 7, suction filtered and freeze-dried to obtain a white solid of 4-hydroxyethylbenzophenone, the molecular structure of which was shown below. The H NMR and IR spectra of the molecule are shown in figure 2 , 3 .
[0066]
Embodiment 2
[0068] Add 10ml of DMF solution with 5.0 parts of 4-hydroxyethyl benzophenone and 0.2 parts of glacial acetic acid in DMF solution into a quartz colorimetric tube, then add 30.0 parts of isopropanol, and react under a 315nm ultraviolet lamp 6d. After the reaction, the mixed solution was poured into distilled water to precipitate, filtered with suction and freeze-dried to obtain a white powder. Purified by silica gel chromatography, the eluent is petroleum ether / ethyl acetate=1:1 (v / v), the corresponding eluate is collected and the solvent is evaporated under reduced pressure to obtain 1,2-bis(4-(2-hydroxy Ethoxy)phenyl)-1,2-diphenylethane-1,2-diol, the molecular structure is as follows. The H NMR and IR spectra of the molecule are shown in Figure 4 , 5 .
[0069]
Embodiment 3
[0071] Under an argon atmosphere, add 5.0 parts of polytetrahydrofuran PTMEG1000 (number-average molecular weight: 1000) into a 250ml three-necked flask with mechanical stirring, heat up to 60°C to melt PTMEG1000, add 100.0 parts of DMF solvent, 1.1 parts of six sub- Methyl diisocyanate trimer, 1.3 parts of hexamethylene diisocyanate monomer and 0.005 part of dibutyltin dilaurate catalyst, add 1.2 parts of compound obtained by embodiment 2 after reacting 5h at 60 ℃ (due to the benzene ring Steric hindrance, the activity of the hydroxyl group on the C-C bond in the center of aromatic pinacol is very weak, and basically does not participate in the reaction, and the two primary hydroxyl groups at the end actually participate in the polymerization), after reacting at room temperature for 24 hours, add 0.25 parts of triethanolamine to continue the reaction After 12 hours, the cross-linked polyurethane containing aromatic pinacol structure was obtained by precipitation in methanol so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com