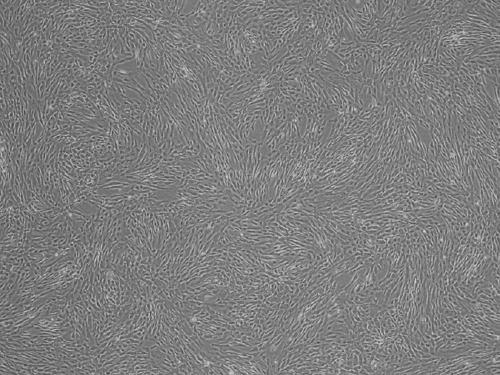
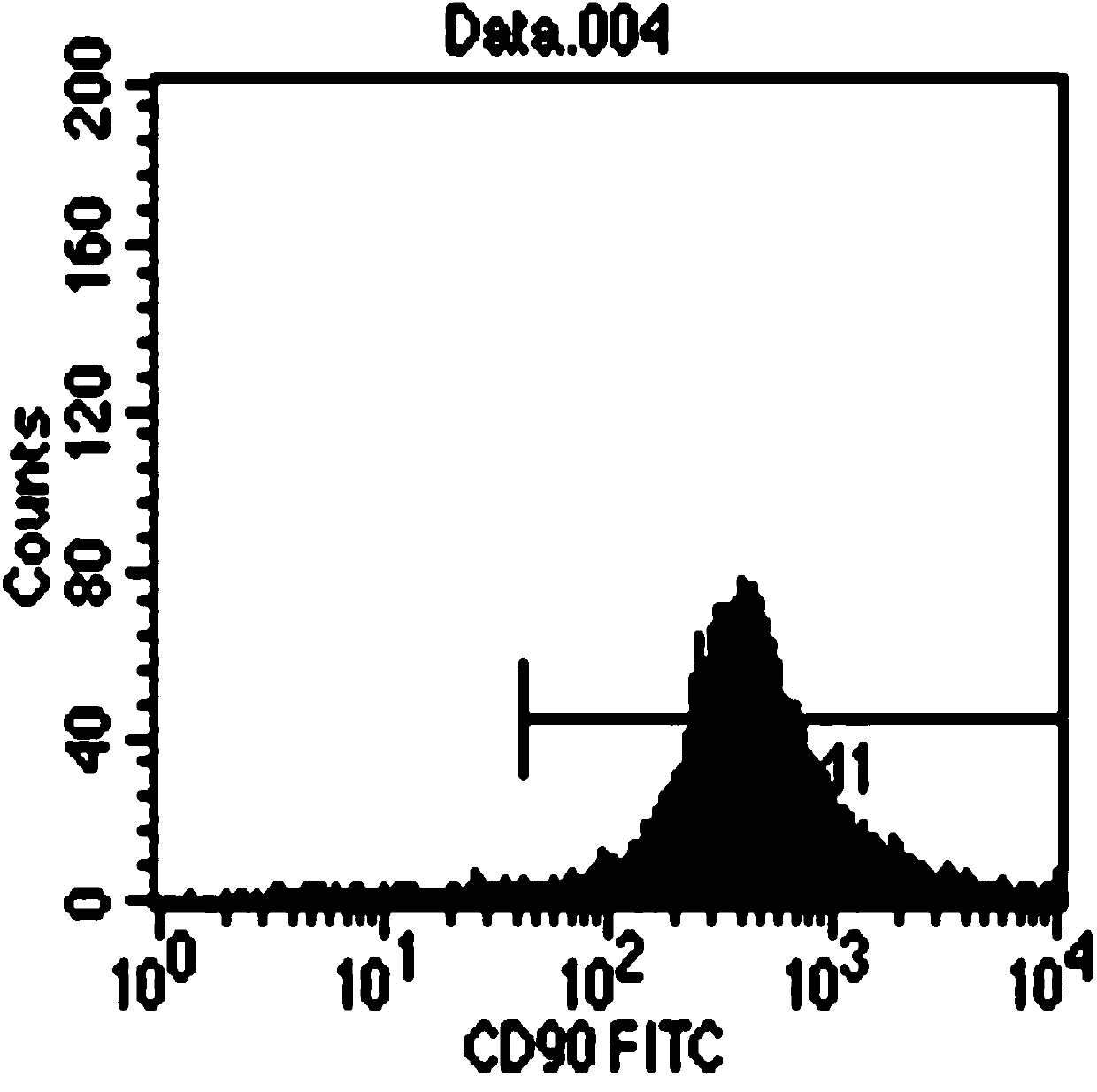
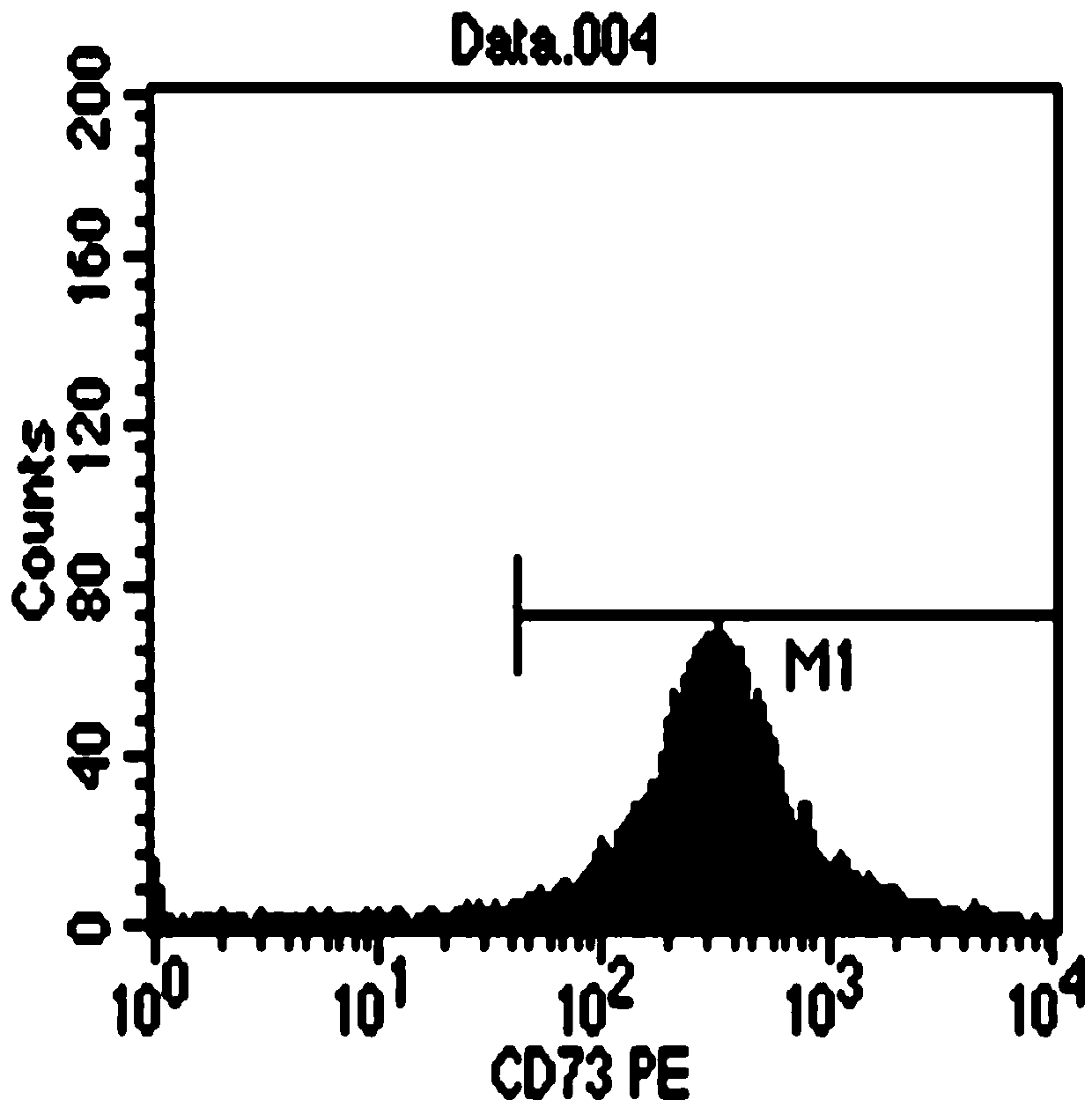
A kind of stem cell culture fluid and injection
A technology of injection and culture solution, applied in the direction of cell culture active agent, culture process, tissue culture, etc., can solve the problems of not being able to meet the needs of cell nutrition, cell activity and secretion factor content, and single nutritional structure, so as to maintain the activity of stem cells , alleviating the sub-health state of the body, and the effect of huge application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for preparing umbilical cord mesenchymal stem cell culture fluid and injection, comprising the following steps.
[0037] Step 1. Sample collection.
[0038] With the informed consent of the puerpera, fresh aseptic umbilical cord tissue was collected from full-term healthy fetuses, stored at 4°C, and processed within 24 hours. Maternal blood should be collected within seven days before delivery for HIV testing, hepatitis B testing, hepatitis C testing, syphilis testing, alanine aminotransferase testing, cytomegalovirus testing and mycoplasma testing. Fresh sterile umbilical cord tissue from the fetus can only be used after all tests pass.
[0039] Cleaning and disinfection: Take out the umbilical cord in the biological safety cabinet, first use 10ml of 0.9% sodium chloride injection to clean the surface, then immerse the umbilical cord in 75% alcohol sterilized by 0.22μm filter for 2 minutes; finally use 0.9% chlorine Sodium chloride injection 10ml was washed a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com