Functionalized hollow mesoporous silica nanospheres and its preparation method and application in adsorption of heavy metal ions
A technology of mesoporous silica and nano-microspheres, used in chemical instruments and methods, alkali metal compounds, adsorption water/sewage treatment, etc., can solve the problem of long time required for adsorption equilibrium, unfavorable repeated use, and small specific surface area. and other problems, to achieve the effect of large industrial application potential, easy separation and recycling, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of functionalized hollow mesoporous silica nanosphere heavy metal ion adsorbent
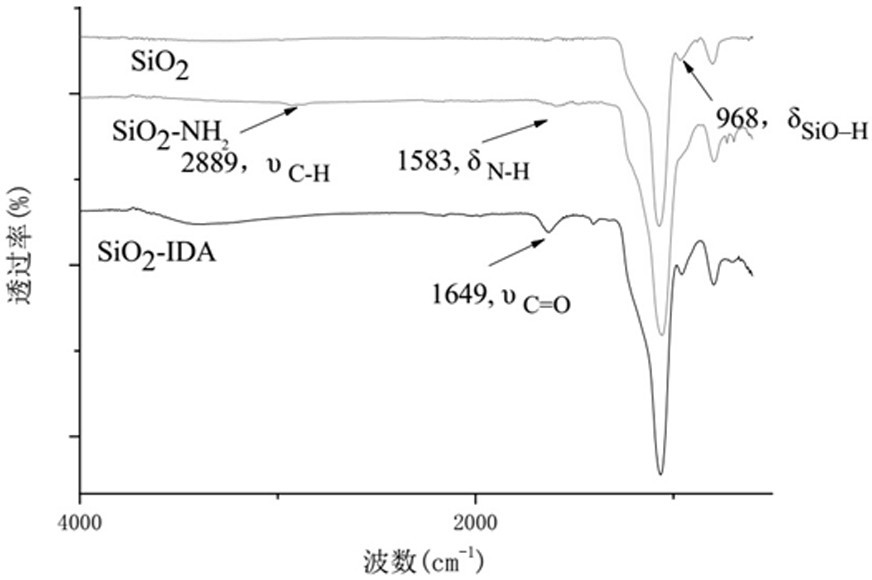
[0034] (1) Hollow mesoporous SiO 2Amino modification of the surface of nanospheres
[0035] 6 g of hollow mesoporous 1500 nm SiO 2 Nanospheres (with a specific surface area of 1440 m 2 / g, with a pore size of 3 nm) was uniformly dispersed in 300 mL of anhydrous toluene by ultrasonication, and 6 g of 3-aminopropyltriethoxysilane coupling agent (KH550) was slowly added dropwise, stirred at room temperature for 2 h, and then placed in N 2 Refluxed in the atmosphere for 18 h, the solid obtained by centrifugation was dispersed in absolute ethanol, washed three times, and then dried in vacuum to obtain modified hollow mesoporous silica nanospheres, which were denoted as hollow mesoporous SiO2. 2 -NH 2 Nanospheres, referred to as SiO 2 -NH 2 nanospheres;
[0036] (2) Hollow mesoporous SiO 2 - Preparation of IDA nanospheres
[0037] 6 g of hollow mesoporous SiO 2...
Embodiment 2
[0063] The preparation method of the functionalized hollow mesoporous silica nanosphere heavy metal ion adsorbent as described in Example 1, the difference is that the hollow mesoporous SiO used in step (1) 2 The particle size of the nanosphere is 200nm (the specific surface area is 680 m 2 / g, with a pore size of 1.1 nm).
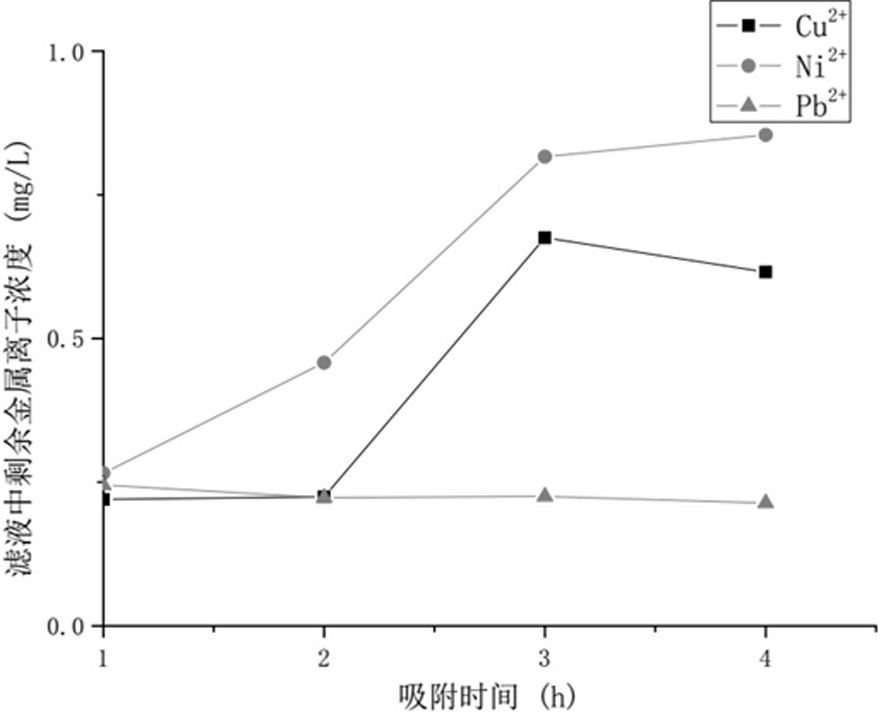
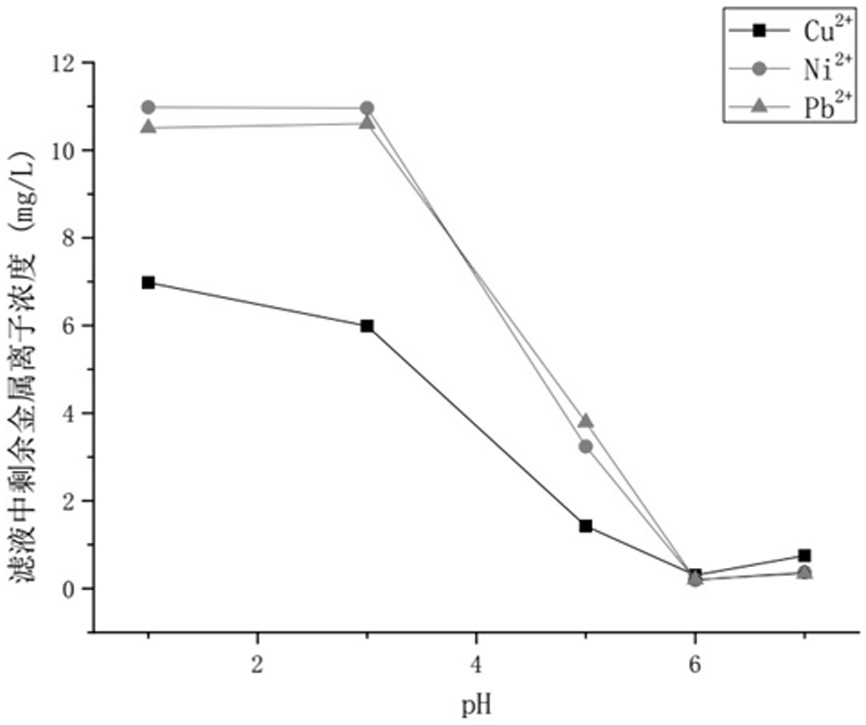
[0064] After testing, the saturated adsorption capacities (mg / g) of the functionalized hollow mesoporous silica nanospheres for copper, nickel, cadmium, and lead were 126.2, 121.2, 129.1, and 125.1, respectively; 2+ 、Ni 2+ 、Cd 2+ Concentration (mg L -1 ) were 0.094, 0.015, 0.091, respectively, and the calcium and magnesium ions were almost unchanged; after desorption and re-adsorption with hydrochloric acid for 5 cycles, the adsorption performance for copper and cadmium still maintained high, reaching more than 96% of the first adsorption, indicating that the material It has good stability and can be recycled.
Embodiment 3
[0066] The preparation method of the functionalized hollow mesoporous silica nanosphere heavy metal ion adsorbent as described in Example 1, the difference is that the hollow mesoporous SiO used in step (1) 2 The particle size of the nanosphere is 500nm (the specific surface area is 920 m 2 / g, with a pore size of 1.9 nm).
[0067] After testing, the saturated adsorption capacities (mg / g) of the functionalized hollow mesoporous silica nanospheres on copper, nickel, cadmium, and lead were 125.4, 120.6, 129.6, and 124.3, respectively; 2+ 、Ni 2+ 、Cd 2+ Concentration (mg L -1 ) were 0.091, 0.018, and 0.090, respectively, and there was almost no change in calcium and magnesium ions; after desorption and re-adsorption with hydrochloric acid for 5 cycles, it still maintained a high adsorption performance for copper and cadmium, reaching more than 96% of the first adsorption, indicating that the material It has good stability and can be recycled.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com