Method for preparing aromatic hydrocarbon by methyl alcohol at high selectivity
A high-selectivity, methanol technology, applied in the field of aromatics, can solve the problems of catalyst promoter metal sintering, reduced catalyst activity, poor catalyst stability, etc., to achieve the effect of promoting the selectivity of aromatics, improving the selectivity of aromatics, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
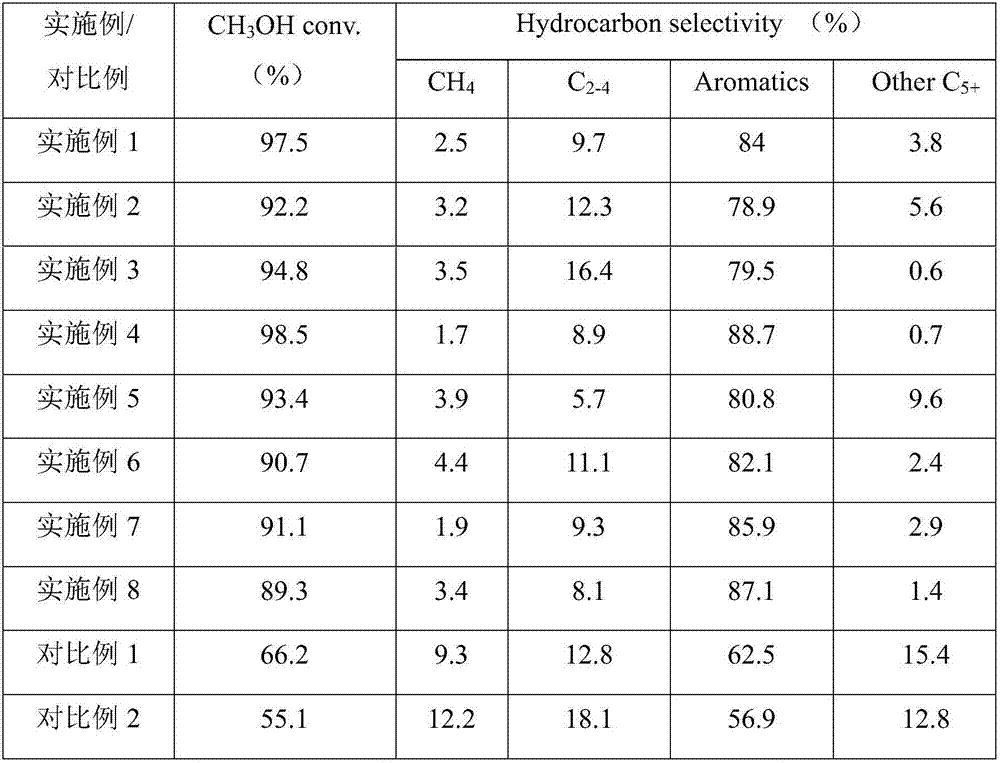
[0027] Weigh 2.0g Cu(NO 3 ) 2 ·3H 2 O, join in 30g absolute ethanol and carry out ultrasonic dispersion, the time is 4h; Weigh the Zn ion-modified H-beta zeolite molecular sieve of 3.0g (Zn content is 0.5wt%), join in the above-mentioned solution, continue ultrasonication 5h; The mixture after ultrasonic dispersion was suction-filtered and washed, and the resulting filter cake was moved to a vacuum drying oven for 24 hours at 80°C; the resulting sample was moved to a tube furnace, and mixed with NO / Ar containing 10% NO by volume gas, heated at a rate of 2°C / min to 550°C for 6 hours; the calcined samples were 2 / Ar atmosphere was reduced for 4 hours at 400°C (heating rate: 1°C / min), and the obtained sample was the catalyst.
[0028] Catalytic reactions are carried out in fixed-bed microreactors. Take 1.0 g of the catalyst and heat it from room temperature to 550 °C at a rate of 5 °C / min in a nitrogen atmosphere, and keep it for 60 min. Then feed methanol and carbon monoxid...
Embodiment 2
[0033] Weigh 3.5g Fe(NO 3 ) 3 9H 2 O, join in 50g water and carry out ultrasonic dispersion, the time is 4h; Weigh the Zr ion modified H-MOR zeolite molecular sieve of 3.0g (Zr content is 0.5wt%), join in the above-mentioned solution, continue ultrasonic 5h; Ultrasonic dispersion After the mixture was suction filtered and washed, the obtained filter cake was moved to a vacuum drying oven and dried at 80°C for 24 h; The rate of 2°C / min was raised to 550°C for 6 hours; the calcined samples were 2 / Ar atmosphere was reduced for 4 hours at 400°C (heating rate: 1°C / min), and the obtained sample was the catalyst.
[0034] The catalytic reaction was carried out in a fixed-bed high-pressure microreactor, and the reaction conditions and product analysis were the same as in Example 1 except that the raw materials were methanol and ethylene (the feed molar ratio was 10:1), and the reaction performance was shown in Table 1.
Embodiment 3
[0036] Weigh 3.0g Co(NO 3 ) 2 ·6H 2 0, join in 45g water and ethanol mixed solution (water and ethanol mass ratio are 1:1) carry out ultrasonic dispersion, the time is 4h; Take by weighing the Cu ion modified H-MCM-22 zeolite molecular sieve of 3.0g (Cu content is 1:1) 0.5wt%), added to the above solution, and continued ultrasonication for 5h; the mixture after ultrasonic dispersion was suction filtered and washed, and the resulting filter cake was moved to a vacuum drying oven and dried at 80°C for 24h; the obtained sample was moved to a tube furnace In the chamber, the mixed gas of NO / Ar containing 10% NO by volume was introduced, and the temperature was raised to 550°C at a rate of 2°C / min for calcination for 6 hours; the calcined samples were 2 / Ar atmosphere was reduced for 4 hours at 400°C (heating rate: 1°C / min), and the obtained sample was the catalyst.
[0037] The catalytic reaction was carried out in a fixed-bed high-pressure microreactor, except that the raw mat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com