Method for recovering 2,4-dinitrophenol hydrogenation reduction byproduct to prepare 2-amino-4-acetamidoanisole
A technology of acetaminoanisole and dinitrophenol, which is applied in the field of preparation of organic compounds, can solve the problems of farmland and water system pollution, unstable product quality, and large amount of impurities, so as to reduce the content, increase the economic benefits of enterprises, The effect of reducing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 5000Kg 2,4-dinitrophenol hydrogenation reduction product is extracted by solid-liquid separation and the mother liquor after extracting the main product 2-amino-4-nitrophenol is concentrated to obtain 750Kg residue;
[0021] Mix 750Kg residue with sulfuric acid and urea, convert 2-amino-4-nitrophenol in the mixture into 5-nitrobenzoxazolone and remove it to obtain acidic acid containing 2-nitro-4-aminophenol The aqueous solution was further processed to obtain 330Kg of 2-nitro-4-aminophenol crude product with a purity of 97.8%;
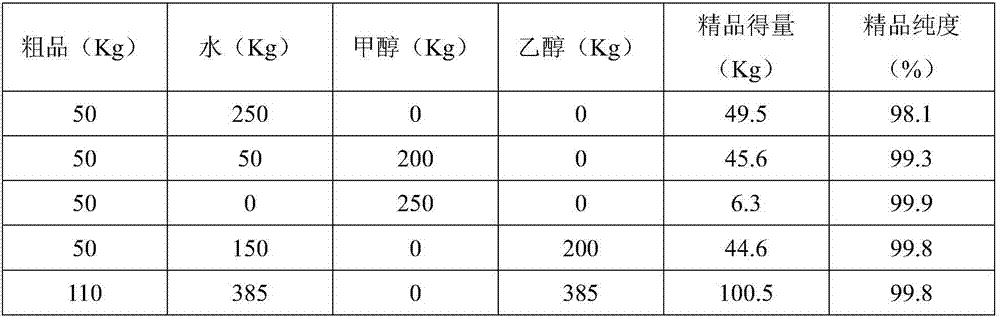
[0022] The 2-nitro-4-aminophenol with a purity of 97.8% obtained by the above method is recrystallized by different solvents respectively, and the results are shown in the following table respectively,
[0023]
Embodiment 2
[0025] The 15.4Kg purity obtained through the steps of Example 1 is that 99.8% 2-nitro-4-aminophenol is mixed with 40Kg mass concentration of 10% liquid caustic soda, and 12.6Kg dimethyl sulfate is added to obtain 15.8Kg 2-nitro -4-Aminoanisole.
[0026] 15.8Kg of 2-nitro-4-aminoanisole was mixed with 95Kg of methanol, 10.1Kg of acetic anhydride, and 5.5Kg of sodium carbonate to obtain 19.2Kg of 2-nitro-4-acetamidoanisole.
[0027] 19.2Kg 2-nitro-4-acetamidoanisole and 70Kg methanol, 10Kg water, 1.0Kg FeCl 3 .6H 2 O mixing, adding 6.3Kg hydrazine hydrate and mixing, obtaining 16.8Kg purity is 99.3% 2-amino-4-acetamidoanisole.
Embodiment 3
[0029] 100.5Kg of 2-nitro-4-aminophenol with a purity of 99.8% obtained through the steps of Example 1 was mixed with 66.9Kg of triethylamine and 500Kg of chloroform, and 51Kg of methylene chloride was introduced to obtain 105.8Kg of 2-nitro-4-aminophenol 4-Aminoanisole.
[0030] 105.8Kg 2-nitro-4-aminoanisole is mixed with 50Kg water, 600Kg ethanol, 49.1Kg acetyl chloride, 62.5Kg N-methylpiperidine to obtain 129.6Kg 2-nitro-4-acetamidoanisole .
[0031] 129.6Kg of 2-nitro-4-acetamidoanisole, 80Kg of methanol, 0.4Kg of Raney nickel, and hydrogen gas were introduced to obtain 108.8Kg of 2-amino-4-acetamidoanisole with a purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com