Linear double-branch azobenzene/graphene composite material and preparation method and application thereof
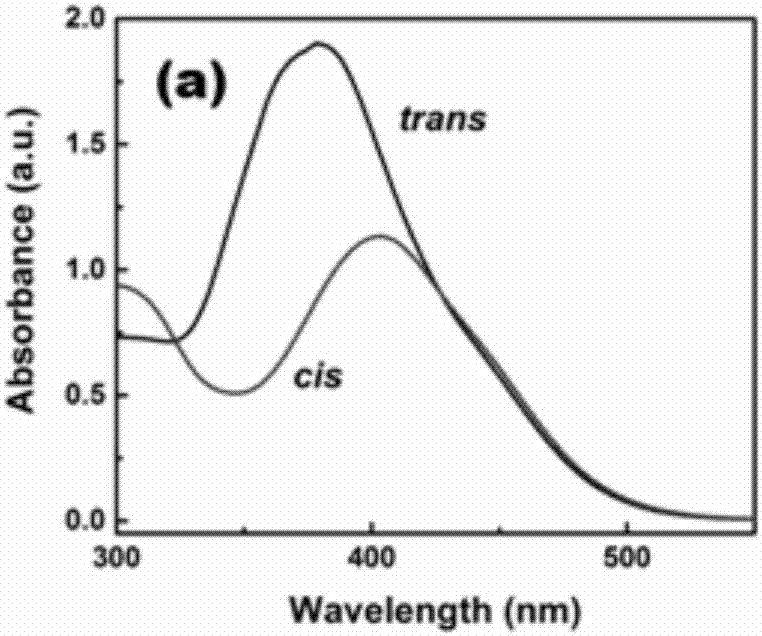
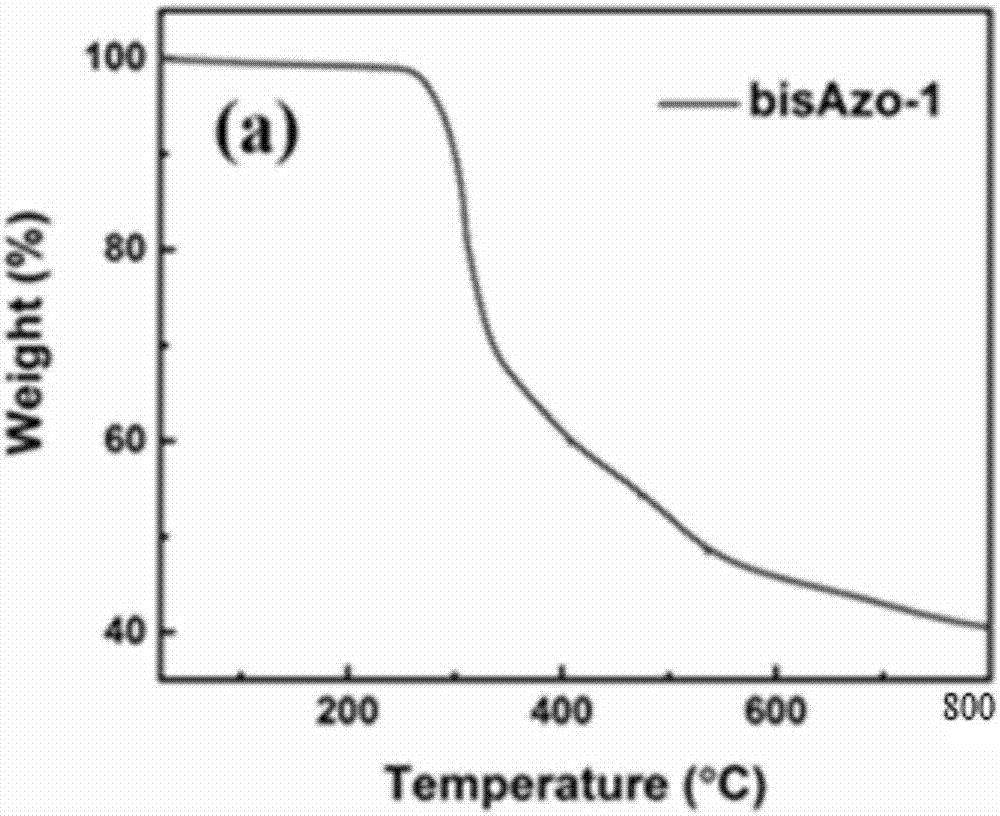
A double-branched azobenzene and composite material technology, applied in the field of composite materials, can solve the problems of no amazing results in the field of solar thermal storage, unsatisfactory energy storage effect, low energy density, etc., achieve long half-life, increase grafting efficiency, high energy storage density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
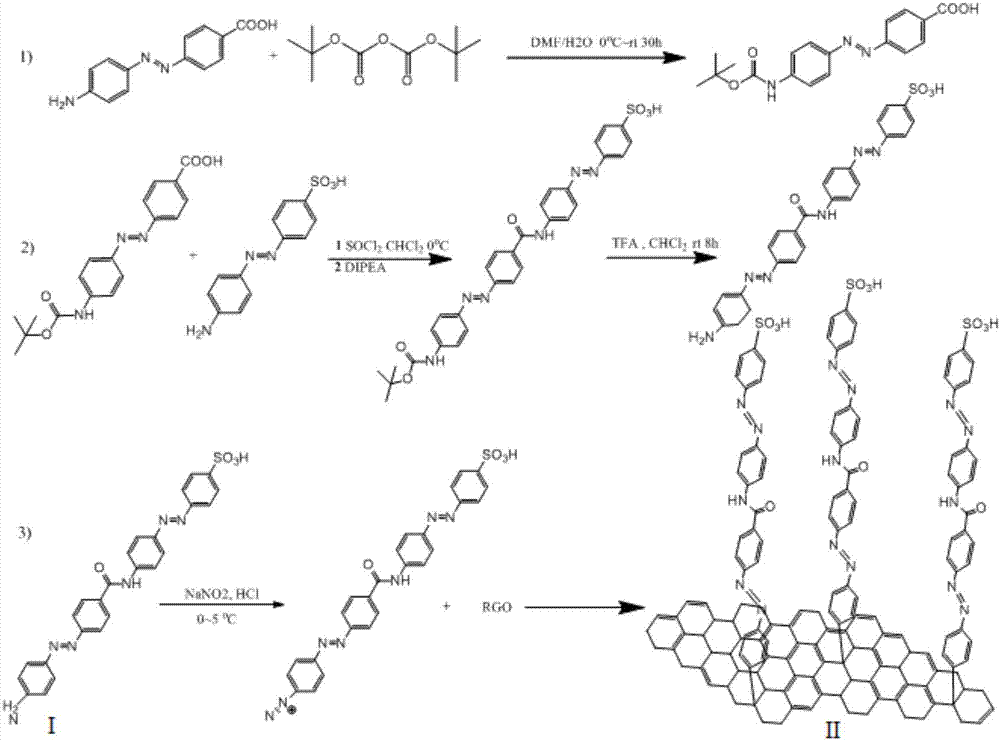
[0035] 1) Dissolve 2.740g of 4-aminobenzoic acid and 1.380g of sodium nitrite in 50ml of deionized water, then slowly add 40ml of 2mol / L hydrochloric acid aqueous solution and place it in an ice bath and stir to prepare the diazonium salt. The prepared diazonium salt solution was slowly added dropwise into 20 mmol of aniline hydrochloric acid aqueous solution under ice bath condition, the pH value was adjusted to 6-7, stirred in ice bath for 6 h under the protection of argon, and reacted overnight. The obtained crude product was washed several times with deionized water and absolute ethanol, and then chromatographic separation was performed using 5 μm silica gel as a stationary phase and ethyl acetate / n-hexane as an eluent. The fraction obtained was distilled under reduced pressure and dried in vacuo to obtain 4-((4-phenylaniline)diazenylbenzoic acid. With 12mmol 4-((4-phenylaniline)diazenylbenzoic acid and 13mmol NaOH Add to 30ml 1:1DMF / H 2 O mixed solution. 12.5 mmol of di...
Embodiment 2
[0039]1) Dissolve 2.740g of 4-aminobenzoic acid and 1.380g of sodium nitrite in 50ml of deionized water, then slowly add 40ml of 2mol / L hydrochloric acid aqueous solution and place it in an ice bath and stir to prepare the diazonium salt. The prepared diazonium salt solution was slowly added dropwise into 20 mmol of aniline hydrochloric acid aqueous solution under ice bath condition, the pH value was adjusted to 6-7, stirred in ice bath for 6 h under the protection of argon, and reacted overnight. The obtained crude product was washed several times with deionized water and absolute ethanol, and then chromatographic separation was performed using 5 μm silica gel as a stationary phase and ethyl acetate / n-hexane as an eluent. The fraction obtained was distilled under reduced pressure and dried in vacuo to obtain 4-((4-phenylaniline)diazenylbenzoic acid. With 12mmol 4-((4-phenylaniline)diazenylbenzoic acid and 13mmol NaOH Add to 30ml 1:1DMF / H 2 O mixed solution. 12.5 mmol of di-...
Embodiment 3
[0043] 1) Dissolve 2.740g of 4-aminobenzoic acid and 1.380g of sodium nitrite in 50ml of deionized water, then slowly add 40ml of 2mol / L hydrochloric acid aqueous solution and place it in an ice bath and stir to prepare the diazonium salt. The prepared diazonium salt solution was slowly added dropwise into 20 mmol of aniline hydrochloric acid aqueous solution under ice bath condition, the pH value was adjusted to 6-7, stirred in ice bath for 6 h under the protection of argon, and reacted overnight. The obtained crude product was washed several times with deionized water and absolute ethanol, and then chromatographic separation was performed using 5 μm silica gel as a stationary phase and ethyl acetate / n-hexane as an eluent. The fraction obtained was distilled under reduced pressure and dried in vacuo to obtain 4-((4-phenylaniline)diazenylbenzoic acid. With 12mmol 4-((4-phenylaniline)diazenylbenzoic acid and 13mmol NaOH Add to 30ml 1:1DMF / H 2 O mixed solution. 12.5 mmol of di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com