Preparation of recombinant protein of long oyster cell wall hydrolase and application of the obtained recombinant protein
A technology of recombinant protein and oyster, which is applied in the fields of molecular biology and biochemistry, and achieves the effects of potential application value, simple genetic manipulation, and favorable expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
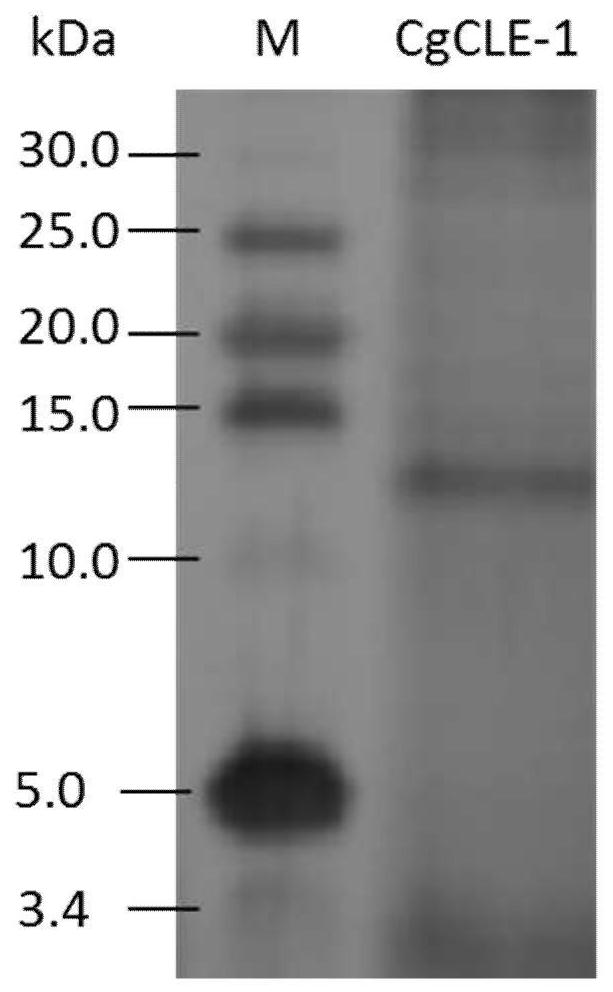
[0028] Experimental example 1: Prokaryotic recombinant expression and purification of long oyster CgCLE-1 in vitro
[0029] 1. Construction of recombinant vector
[0030] The recombinant vector used in the present invention is the pPICZαA prokaryotic expression vector of Invitrogen Company. Total RNA was extracted from fresh long oyster hemolymphocytes, and cDNA was obtained by reverse transcription PCR. By PCR technology, using primers P1 (5'-GAATTCAGAGGAGAACCGATGGAAGCTA-3') and P2 (5'-GCGGCCGCCACAAAGTACAGACCCCGAGTT-3') with EcoRI and Not I restriction enzyme sites added at the 5' end to amplify the long oyster cell wall Coding region of the hydrolase Hydolase_2 domain. The reaction conditions were as follows: 94°C pre-denaturation for 5 minutes, followed by the following cycles: 94°C denaturation for 30 seconds, 65°C annealing for 30 seconds, 72°C extension for 2 minutes, a total of 35 cycles, and finally 72°C extension for 10 minutes. The amplified fragment was purified ...
experiment example 2
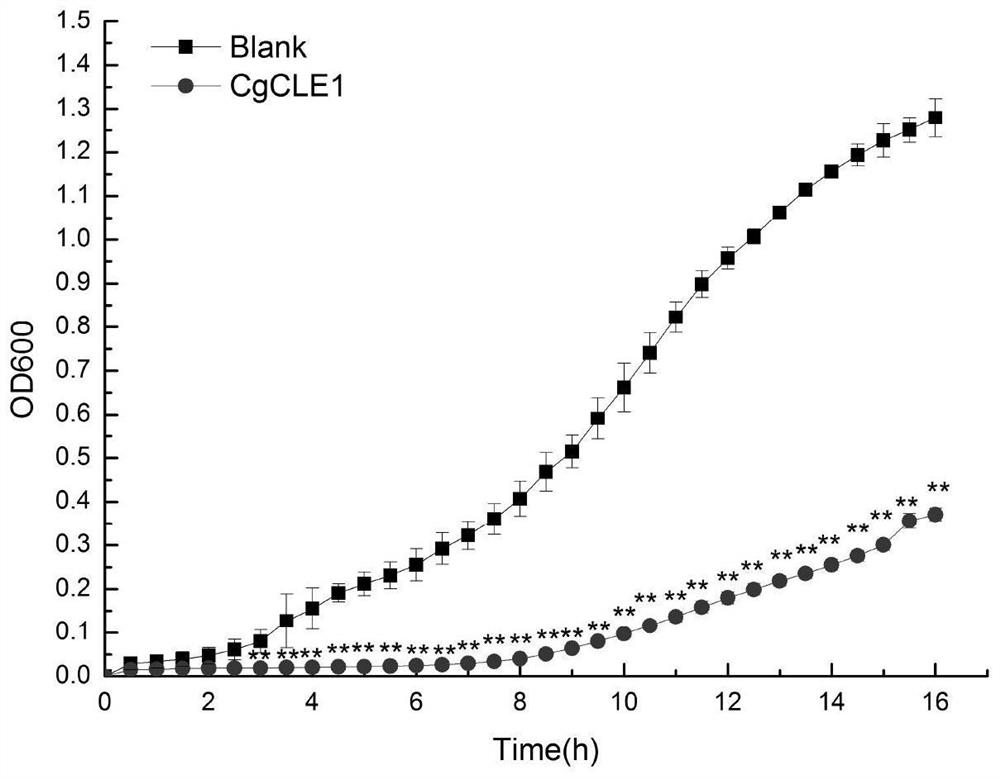
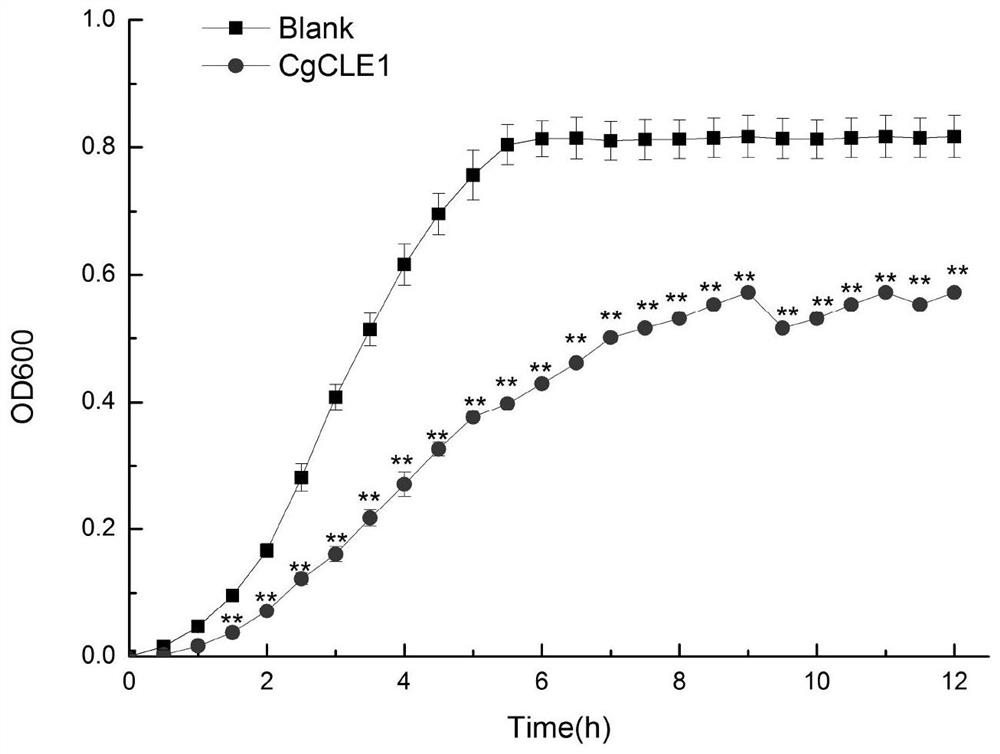
[0034] Experimental Example 2: Antibacterial Activity Detection of CgCLE-1 Recombinant Protein from Long Oyster
[0035]Escherichia coli (Escherichia coli), Aeromonas hydrophila (Aeromonas hydrophila) and Staphyloccocus aureus (Staphyloccocus aureus) were cultured in LB medium at 37°C and 220rpm until logarithmic growth phase; Vibrio splendidus (Vibrio splendidus), Vibrio vulnificus, Vibrio alginolyticus, Pseudomonas putida and Pseudomonas aeruginosa were respectively cultured in 2216E medium at 28°C and 220rpm until logarithmic growth phase The obtained cells were collected by centrifugation and washed with TBS (50mM Tris-HCl, 100mM NaCl, pH=7.4) and resuspended to a concentration of 10 4 CFU. 50 μ L above-mentioned embodiment obtains recombinant protein CgCLE-1 (160 μ g mL -1 ) and an equal volume of each bacterial suspension obtained above were incubated at room temperature for 2 h. In blank group (Blank group), 50 μL TBS was used instead of recombinant protein. Take 20...
experiment example 3
[0036] Experimental results: CgCLE-1 can significantly inhibit Vibrio splendidus ( figure 2 ), Escherichia coli ( image 3 ), Vibrio vulnificus ( Figure 4 ), Pseudomonas putida ( Figure 5 ), Vibrio alginolyticus ( Figure 6 ), Pseudomonas aeruginosa ( Figure 7 ), Aeromonas hydrophila ( Figure 8 ) and Staphylococcus aureus ( Figure 9 ) growth (P<0.01). Experimental Example 3: Determination of the minimum inhibitory concentration (MIC) of CgCLE-1 recombinant protein of long oyster
[0037] The CgCLE-1 recombinant protein was diluted in multiples, and the antibacterial activity was tested according to the method of Experimental Example 2. The lowest drug concentration that can inhibit the obvious growth of a certain microorganism is the MIC of CgCLE-1.
[0038] Experimental results: See Table 1 for the minimum inhibitory concentrations of CgCLE-1 on the tested bacteria.
[0039] Table 1. The minimum inhibitory concentration of CgCLE-1 against different bacteria
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com