Hydroxyethyl cellulose/soybean protein composite sponge with water sensitive shape memory function and preparing method of composite sponge
A technology of hydroxyethyl cellulose and soybean protein, which is applied in the field of biomedical materials, can solve the problems such as no composite sponge material of hydroxyethyl cellulose and soybean protein isolate, no three-dimensional scaffold material without shape memory function, etc. Achieving the effects of good biocompatibility, low cost and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
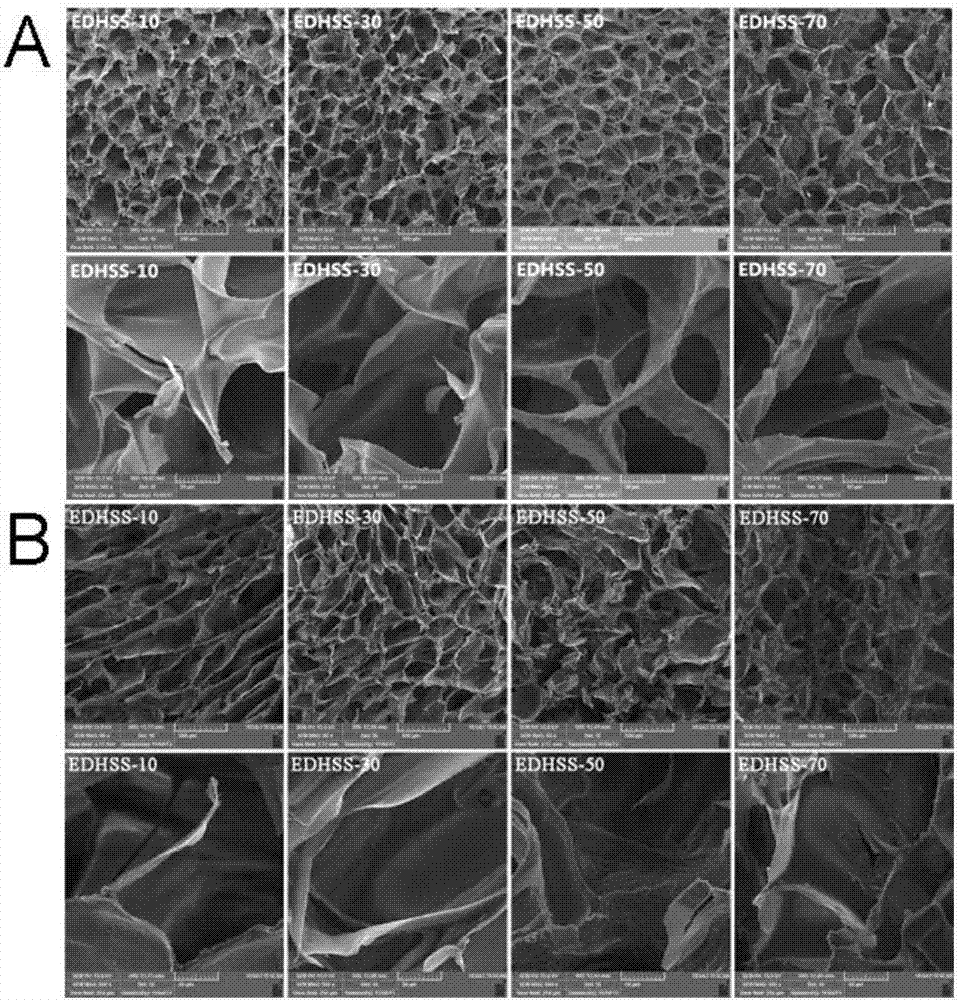
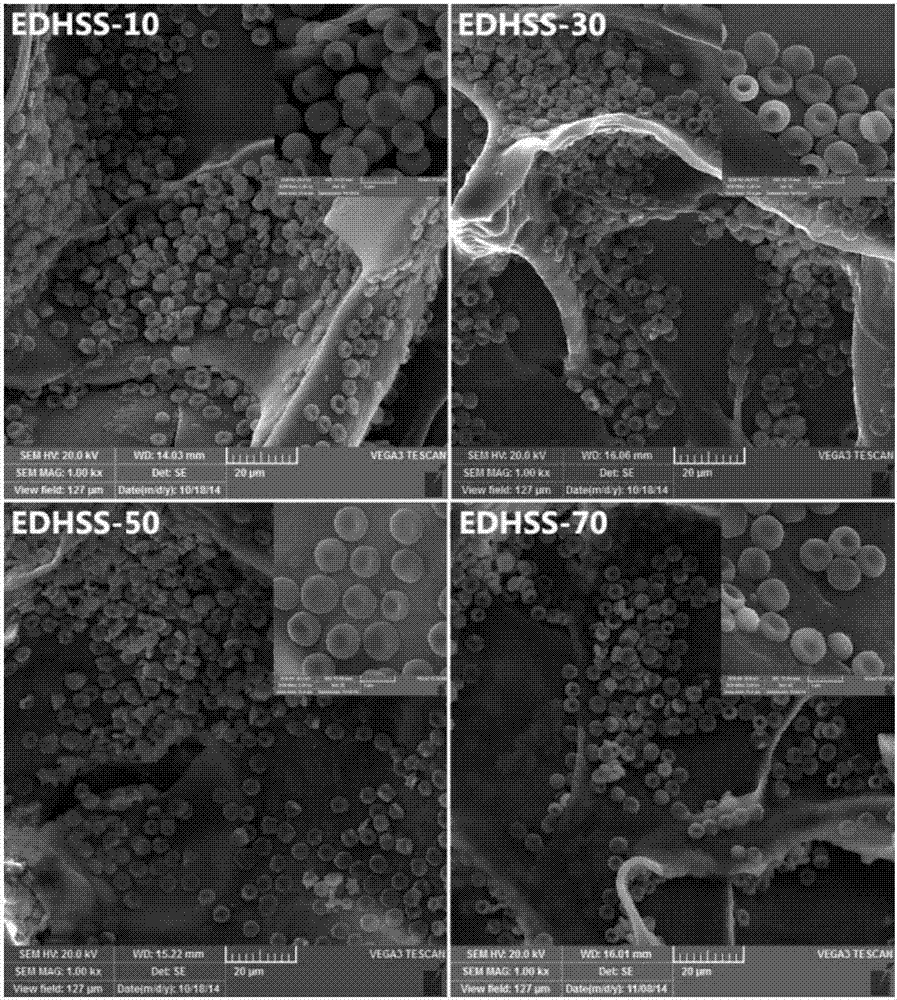
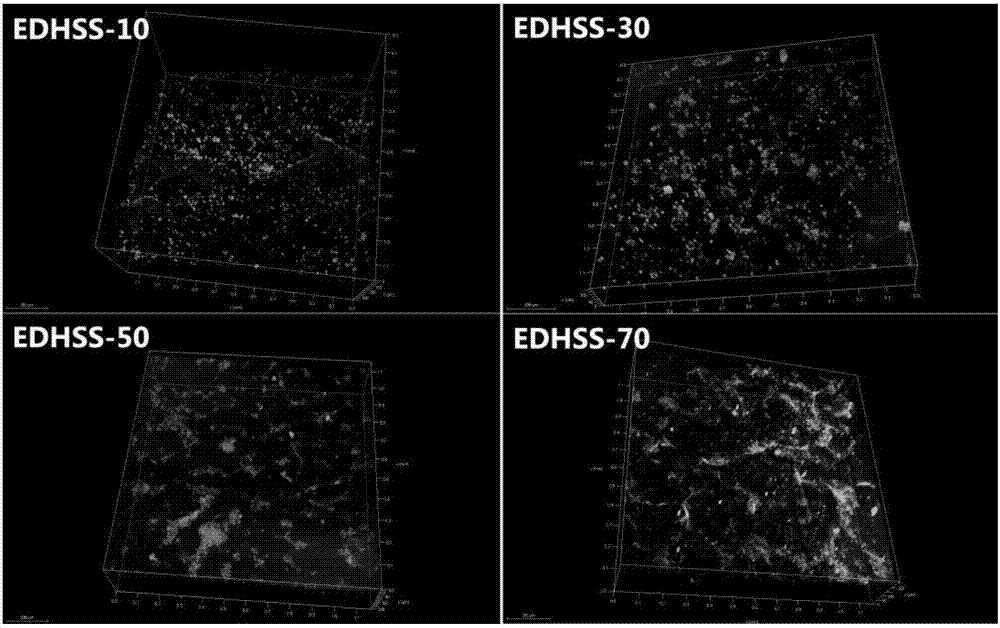
[0025] Hydroxyethylcellulose was dissolved in deionized water at a concentration of 2%. Soy protein isolate was dissolved in 5% NaOH solution with a concentration of 10%. The two solutions are mixed evenly, and the dry weight ratio of soybean protein isolate to hydroxyethyl cellulose / soy protein isolate is between 10% and 70%, and cross-linked with ethylene glycol diglycidyl ether (EDGE). EDGE accounts for 50% of the dry weight of hydroxyethyl cellulose / soy protein isolate, stir and mix at room temperature for 30 minutes, and centrifuge to remove air bubbles. The solution was poured into a mold, firstly frozen at -20°C for 12 hours, then frozen at -80°C for 24 hours, and then placed in a freeze-vacuum drying oven to dry and shape. Cut the composite sponge into 1×1cm sheet, freeze it in liquid nitrogen for 10 minutes, then observe the surface structure of the composite sponge with a scanning electron microscope, then cut the composite sponge into a size of 1×2cm, and wrap it w...
Embodiment 2
[0028] The material prepared in Example 1 is cut into sheets of 1 * 1 * 0.2cm size, and each concentration (the dry weight ratio of hydroxyethyl cellulose / soy protein isolate that soybean protein isolate accounts for is 10%, 30%, 50%, 70%) materials, cut 3 pieces, washed three times with normal saline, then added 10mL of normal saline, kept at 37°C for 30min, and set aside; 4mL of blood was collected from the heart of a New Zealand rabbit, and mixed with normal saline Mix well at a ratio of 1:1.25, then add 200uL of diluted blood to each material soaked in normal saline, and conduct positive and negative control tests at the same time, with deionized water as the positive control and normal saline as the negative control. After 30 minutes, it was centrifuged at 1500 rpm for 10 minutes, and the absorbance value was measured at 545 nm of ultraviolet light by an ultraviolet spectrophotometer to calculate the hemolysis rate.
[0029] The following table 1 is the hemolysis test res...
Embodiment 3
[0034] The sponge material prepared in Example 1 was cut into thin pieces of 1 cm×1 cm×0.3 cm, wrapped in tin foil and marked, and autoclaved at 121° C. for 21 minutes. Remove the autoclaved samples and place them separately in 24-well cell culture plates. 1 mL of cell suspension containing 10% serum at a certain concentration was dropped on the surface of the composite material, and cells at the same concentration and volume were added to blank wells as a negative control group. Put in 37°C CO 2 The cells were cultured in the incubator for 72 hours, and the liquid was replaced at 48 hours. After 72 hours, the 24-well plate was taken out, the culture medium was sucked out, rinsed with PBS for 3 times, and then fixed with an appropriate amount of 4wt% formaldehyde solution at 4°C overnight, then the liquid was sucked out and washed with PBS for 3 times, and the prepared 10ug was added / ml of DiO cell membrane fluorescent staining solution, stained in a dark room for 15-20min,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com