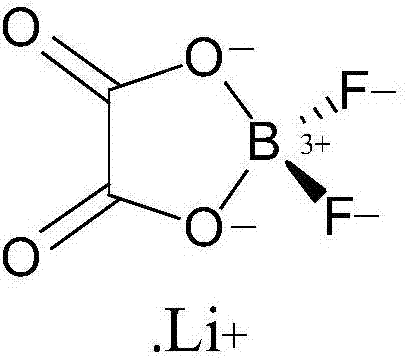
Process for synthesizing lithium difluoro(oxalate)borate from lithium bis(oxalate)borate
The technology of lithium difluorooxalate borate and lithium bisoxalate borate is applied in the field of synthesis technology of electrolyte lithium salt, can solve the problems of difficult separation of by-products, low reaction yield and high requirements for reaction equipment, and achieves great implementation value and social economy. Benefit, mild preparation conditions, less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0032] As an embodiment of the present invention, the compound containing fluorine, boron, and lithium is lithium tetrafluoroborate, fluoroboric acid, boron trifluoride dimethyl carbonate complex, lithium fluoride, boron trifluoride, trifluoride Boron trifluoride acetonitrile, boron trifluoride ether, boron trifluoride dimethyl carbonate, boron trifluoride ethyl acetate, boron trifluoride tetrahydrofuran, boron trifluoride methyl ether complex, boron trioxide, borax , boric acid, lithium carbonate, lithium oxalate, lithium chloride, lithium hydrogen oxalate, lithium hydroxide, lithium sulfate, lithium dihydrogen phosphate, lithium fluoride, lithium oxide, lithium bromide, lithium amide, lithium diisopropylamide one or a combination.
[0033] As a preferred form of the present invention, the compound containing fluorine, boron, and lithium is lithium tetrafluoroborate, fluoroboric acid, lithium fluoride, boron trifluoride, boron trifluoride acetonitrile, boron trifluoride ether...
Embodiment approach 1
[0040] Embodiment 1: This embodiment provides a synthesis process for preparing lithium difluorooxalate borate with lithium bisoxalate borate. The synthesis process includes the following steps:
[0041] 1), mixing lithium bisoxalate borate with compounds containing fluorine, boron, and lithium in proportion to obtain a mixture;
[0042] 2) Add the mixture in step 1) to the solvent, and react at 0-150° C. with a reaction pressure of 101kpa-150kpa to obtain the product, which is crystallized and vacuum-dried to obtain lithium difluorooxalate borate.
Embodiment approach 2
[0043] Embodiment 2: The synthesis process as described in Embodiment 1, the compound containing fluorine, boron, and lithium is lithium tetrafluoroborate, fluoroboric acid, boron trifluoride dimethyl carbonate complex, lithium fluoride, three Boron fluoride, boron trifluoride acetonitrile, boron trifluoride ether, boron trifluoride dimethyl carbonate, boron trifluoride ethyl acetate, boron trifluoride tetrahydrofuran, boron trifluoride methyl ether complex, three Diboron oxide, borax, boric acid, lithium carbonate, lithium oxalate, lithium chloride, lithium hydrogen oxalate, lithium hydroxide, lithium sulfate, lithium dihydrogen phosphate, lithium fluoride, lithium oxide, lithium bromide, lithium amide, diisopropyl Any one or combination of lithium amides.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com