Extracting method for separating and purifying heavy rare earth
A heavy rare earth and extraction technology, applied in the direction of improving process efficiency, can solve the problems of high acid and alkali consumption, affecting the separation and high purification of heavy rare earth, and low impurity removal rate, so as to achieve reduced acid and alkali consumption, good interface phenomenon, Effect of Separation Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
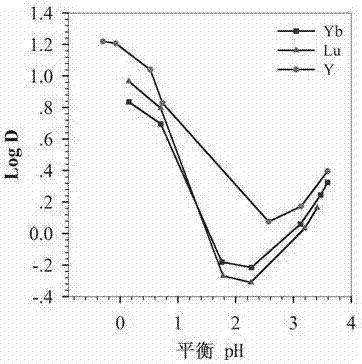
Examples
Embodiment 1
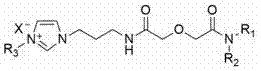
[0026] Weigh 100g of diglycolic anhydride and dissolve it in 1000mL of tetrahydrofuran, add 189g of di-n-octylamine, and react at room temperature for 48 hours under the protection of argon, and dissolve the obtained solution in 400mL of chloroform after rotary evaporation, and wash with dilute hydrochloric acid solution After distillation and vacuum drying, dioctyl diglycol amic acid was obtained; 90g dioctyl diglycol amic acid, 37.2g aminopropyl imidazole, 61.2g dicyclohexylcarbodiimide, 40.2g 1-hydroxybenzene Dissolve triazole in 1000mL chloroform, react at room temperature under the protection of argon for 12h, filter the solution under reduced pressure, distill and dissolve it in 500mL ethyl acetate, wash with sodium carbonate solution to remove residual 1-hydroxybenzo Triazole, spin out ethyl acetate under vacuum condition, adopt silica gel column chromatography, use chloroform and methanol as mobile phase, obtain 2-[2-(aminopropyl imidazole-2 oxo)]-N,N -dioctylacetamide...
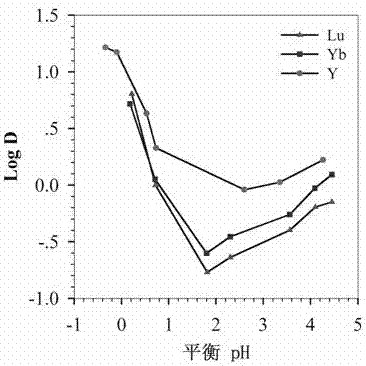
Embodiment 2
[0028] Weigh 100g of diglycolic anhydride and dissolve in 1000mL of tetrahydrofuran, add 189g of di(2-ethylhexyl)amine, and react at room temperature for 48h under the protection of argon, and dissolve the obtained solution in 400mL of chloroform after rotary evaporation, and use After washing with dilute hydrochloric acid solution, distill and vacuum-dry to obtain bis(2-ethylhexyl) diglycol amic acid; 90g bis(2-ethylhexyl) diglycol amic acid, 37.2g aminopropyl imidazole, 61.2 g of dicyclohexylcarbodiimide and 40.2g of 1-hydroxybenzotriazole were dissolved in 1000mL of chloroform, reacted at room temperature under the protection of argon for 12h, and the solution was filtered under reduced pressure, distilled and dissolved in 500mL of ethyl acetate In, wash with sodium carbonate solution to remove residual 1-hydroxybenzotriazole, spin out ethyl acetate under vacuum condition, adopt silica gel column chromatography, use trichloromethane and methanol as mobile phase, obtain 2-[2-...
Embodiment 3
[0030]Weigh 100g of diglycolic anhydride and dissolve it in 1000mL of tetrahydrofuran, add 189g of di-n-octylamine, and react at room temperature for 48 hours under the protection of argon, and dissolve the obtained solution in 400mL of chloroform after rotary evaporation, and wash with dilute hydrochloric acid solution After distillation and vacuum drying, dioctyl diglycol amic acid was obtained; 90g dioctyl diglycol amic acid, 37.2g aminopropyl imidazole, 61.2g dicyclohexylcarbodiimide, 40.2g 1-hydroxybenzene Dissolve triazole in 1000mL chloroform, react at room temperature under the protection of argon for 12h, filter the solution under reduced pressure, distill and dissolve it in 500mL ethyl acetate, wash with sodium carbonate solution to remove residual 1-hydroxybenzo Triazole, spin out ethyl acetate under vacuum condition, adopt silica gel column chromatography, use chloroform and methanol as mobile phase, obtain 2-[2-(aminopropyl imidazole-2 oxo)]-N,N - Dioctyl acetamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com