Application of expired drug aspirin in rechargeable battery
A technology of aspirin and rechargeable batteries, applied in non-aqueous electrolyte battery electrodes, battery electrodes, secondary batteries, etc., can solve problems such as water or soil pollution, drug-resistant fungi, infectious diseases, etc., and achieve stable and reversible cycle charging /Discharge performance, reduce environmental emissions and pollution, and the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The application of the expired medicine aspirin in this embodiment in the sodium ion battery is carried out according to the following steps:
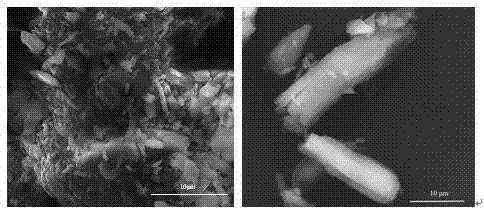
[0027] (1) Grind expired drug aspirin into powder, and use scanning electron microscope to observe its microscopic appearance, as shown in figure 1 shown by figure 1 It can be seen that the microscopic morphology of aspirin, an expired drug, is mainly rod-shaped, accompanied by particles, and the powder, conductive graphite, and polyvinyl alcohol (PVA) are uniformly mixed at a mass ratio of 7:2:1 to form an electrode slurry;
[0028] (2) Apply the electrode slurry obtained in step (1) evenly on the surface of the copper mesh collector, and dry it in vacuum at 60°C for 24 hours to obtain an electrode sheet;
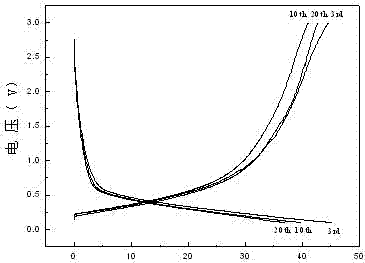
[0029] (3) The electrode sheet prepared in step (2) was used as the working electrode, the sodium foil was used as the counter electrode and the reference electrode, the non-woven fabric was used as the separator, and the e...
Embodiment 2
[0031] The application of the expired drug aspirin in the lithium-ion battery of this embodiment is carried out according to the following steps:
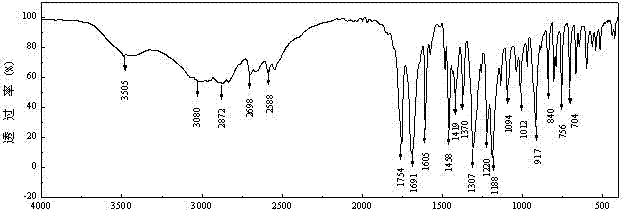
[0032] (1) Grind expired aspirin into powder, and use Fourier transform infrared spectroscopy to test its microstructure, such as figure 2 shown by figure 2 It can be seen that at a wave number of 3080 cm -1 、2872 cm -1 、1754 cm -1 、1691 cm -1 、1605 cm -1 、1458 cm -1 、1095cm -1 、1012 cm -1 corresponding to the stretching vibration of the C-OH bond, -CH 3 The stretching vibration of the bond, the stretching vibration of the C=O bond on the lipid group, the stretching vibration of the C=O bond on the carboxyl group, the stretching vibration of the C=C in the benzene ring, -CH 3 The bending vibration of the C-H bond, the bending vibration of the C-H bond, and the stretching vibration of the C-O bond on the lipid group. Obviously, the main component of the expired drug aspirin is still ethyl o-acetylsalicylate, and the powde...
Embodiment 3
[0036] The application of the expired medicine aspirin in this embodiment in the sodium ion battery is carried out according to the following steps:
[0037] (1) Grind the expired drug aspirin into powder, and evenly mix the powder with acetylene black and polyvinylidene fluoride (PVDF) at a ratio of 6:3:1 to form an electrode slurry;
[0038] (2) Apply the electrode slurry obtained in step (1) evenly on the surface of the nickel foil current collector, and dry it in vacuum at 60°C for 24 hours to prepare an electrode sheet;
[0039] (3) The electrode sheet prepared in step (2) was used as the working electrode, the sodium foil was used as the counter electrode and the reference electrode, the non-woven fabric was used as the separator, and the electrode sheet containing 1 mol / L NaClO 4 A mixture of 5% fluorinated ethylene carbonate, ethylene carbonate (EC) and diethyl carbonate (DEC) is the electrolyte, where the volume ratio of EC to DEC is 1:1, and then filled with high-pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com