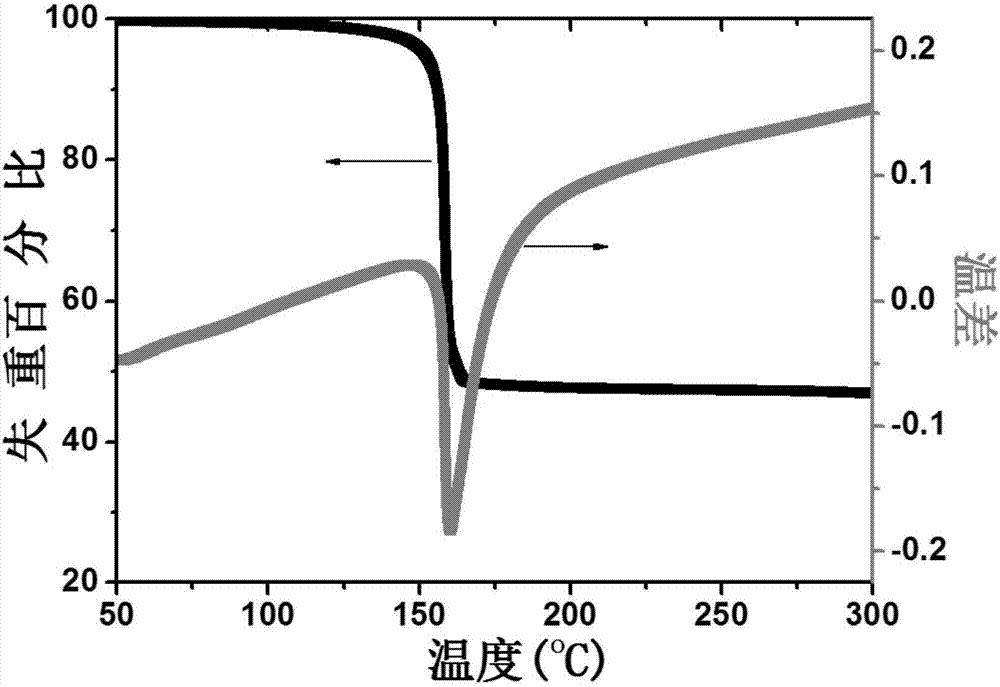
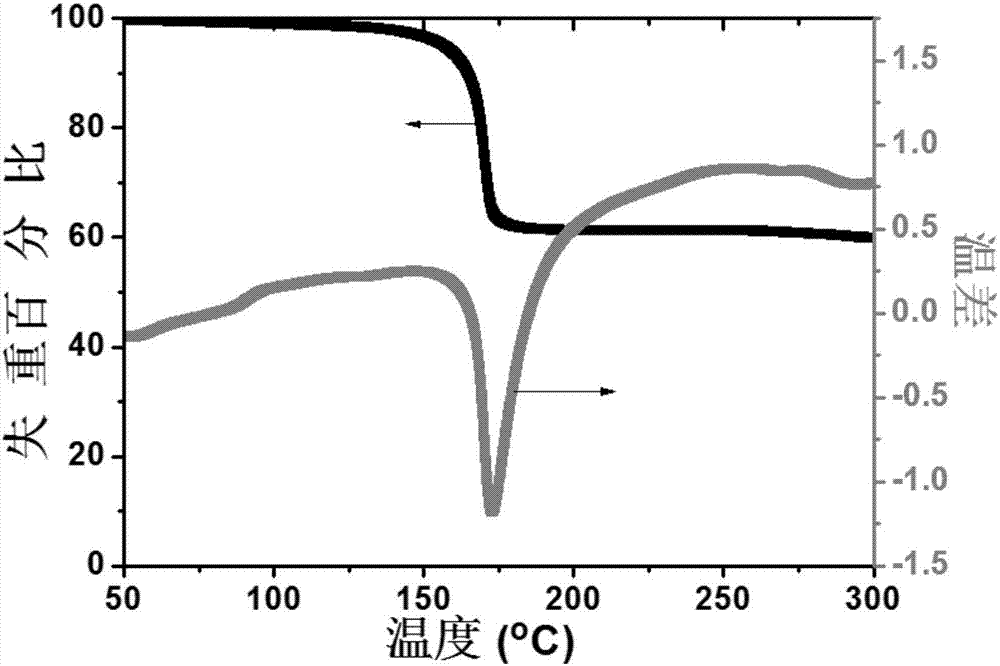
Biphenyl substituted adamantane derivative monomolecular resin, positive photoresist composition and negative photoresist composition
A positive photoresist and negative photoresist technology, applied in optics, optomechanical equipment, instruments, etc., can solve the problem of lithography pattern resolution and edge roughness cannot meet, molecular weight polydispersity, large molecular volume, etc. problem, to achieve the effect of increased interaction, high glass transition temperature, molecular size and single
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of 1,3,5,7-tetra-(3-bromo-4-methoxyphenyl)adamantane, the synthetic route is as follows:
[0062]
[0063] Specific steps:
[0064] Add 5.0g (i.e. 11.0mmol) of tetrabromoadamantane and 20 mL (i.e. 160mmol) of o-methoxybromobenzene into a 250ml three-neck flask equipped with a condenser tube, and connect an inverted funnel to 30% NaOH with a catheter from the upper end of the condenser tube. In aqueous solution, to absorb the HBr produced by the reaction. Under the cold water bath at 5°C, add 1.2g (ie 4.5mmol) of AlBr to the reaction system 3 , continue to stir and react under the cold water bath for 1 hour, then remove the cold water bath, return to room temperature and react for about 3 hours, and finally the reaction system is heated to 60°C in the oil bath for 4 hours, the reaction solution is cooled to room temperature, and poured into 100mL acidic ice Stir in water for 1h. After the ice melted completely, add 100ml of ethyl acetate to the mixt...
Embodiment 2
[0066] The preparation of 1,3,5,7-tetra-(2-methoxy-3',4'-dimethoxy-5-biphenyl)adamantane, the synthetic route is as follows:
[0067]
[0068] Specific steps:
[0069] Under the protection of high-purity nitrogen, add 876.0mg (i.e. 1mmol) of 1,3,5,7-tetra-(3-bromo-4-methoxyphenyl)adamantane, 36.6mg ( ie 0.05mmol) of PdCl 2 (dppf), 910mg (ie 5.0mmol) of 3,4-dimethoxyphenylboronic acid ester and 581mg (ie 10mmol) of potassium fluoride solid, the system was under nitrogen atmosphere, and finally added redistilled dioxane 15ml, the reaction solution was heated to reflux for 6h, cooled to room temperature, and extracted with dichloromethane / water, the organic layers were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, recrystallized in ethyl acetate to obtain 752mg of a white solid, the yield 68%. 1 H NMR (400MHz, CDCl 3 )δ(ppm)7.57(s,4H,benzene),7.36-7.38(m,8H),7.19-7.08(m,8H,benzene),6.95(s,4H,benzene),3.94(s,12H,- ...
Embodiment 3
[0071] The preparation of 1,3,5,7-tetra-(2-hydroxy-3',4'-dihydroxy-5-biphenyl)adamantane, the synthetic route is as follows:
[0072]
[0073] Specific steps:
[0074] Add 1100 mg (ie 1.0 mmol) of 1,3,5,7-tetra-(2-methoxy-3',4'-dimethoxy-5-biphenyl)adamantane into a 250 mL three-necked flask and 50ml of dichloromethane, dissolved under a nitrogen atmosphere, and at a low temperature of -78°C, add 10ml of boron tribromide-containing dichloromethane solution (concentration 0.3g / ml) dropwise to the reaction solution with a syringe, and the reaction solution is at - After reacting at 78°C for 1 hour, the temperature was gradually raised to room temperature, and the reaction was continued for 6 hours. The reaction was quenched by slowly adding 20 ml of ice water to the reaction system, and a large amount of white solid was precipitated. The reaction system was filtered to obtain a white solid, which was washed with water and dichloromethane respectively. The obtained solid was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com