2,5-diphenyl-1,3,4-oxadiazole spirocyclic aromatic hydrocarbon steric hindrance type bipolar light-emitting material and preparation method thereof
A technology of oxadiazole spiro aromatic hydrocarbons and luminescent materials, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., and can solve the problems of reducing the yield rate of device products, increasing the difficulty of device manufacturing, and increasing production costs, etc. , to achieve good photoelectric performance, large steric hindrance effect, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
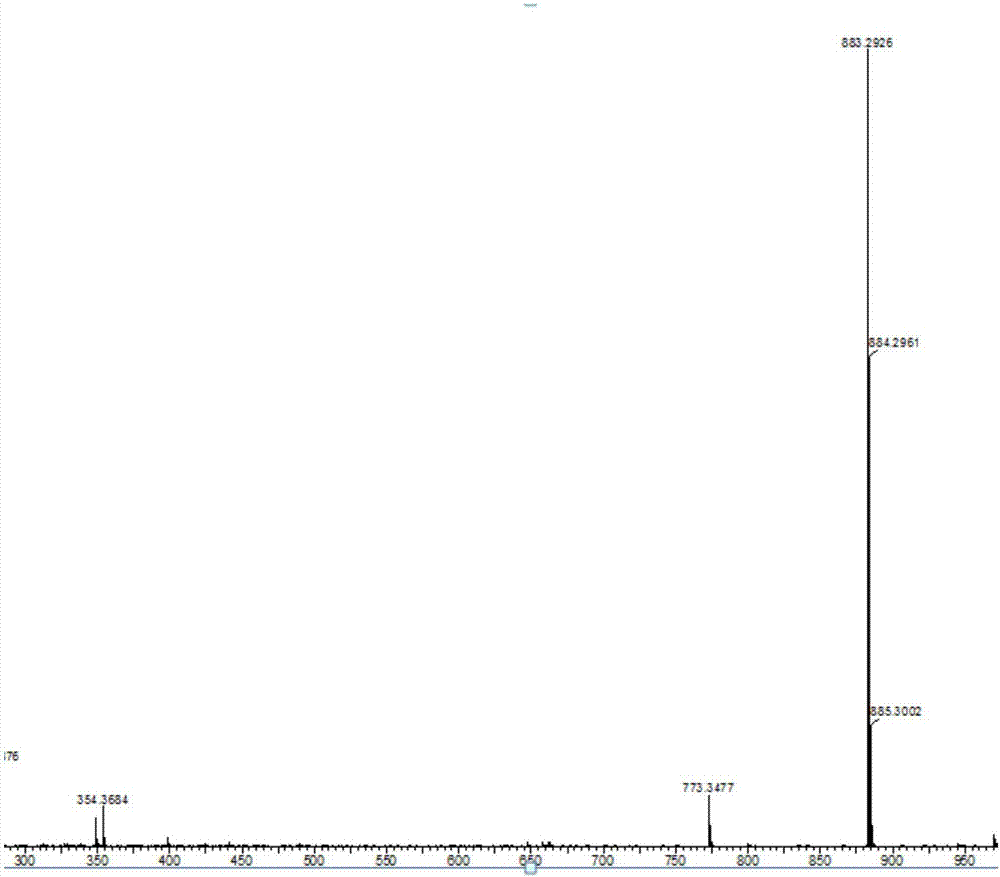
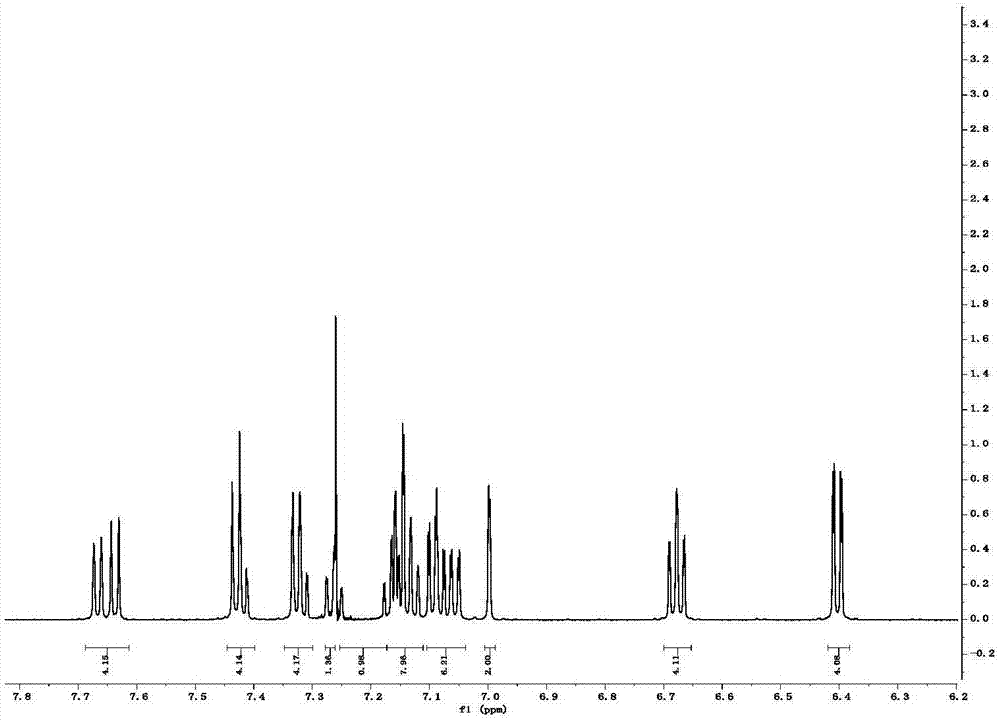
[0038] Example 1: Synthesis of 2,5-bis(2-(spiro-9,9'-oxanthrene fluorene)phenyl)-1,3,4--oxadiazole
[0039] The first step: 2,5-bis(2-bromophenyl)-1,3,4--oxadiazole
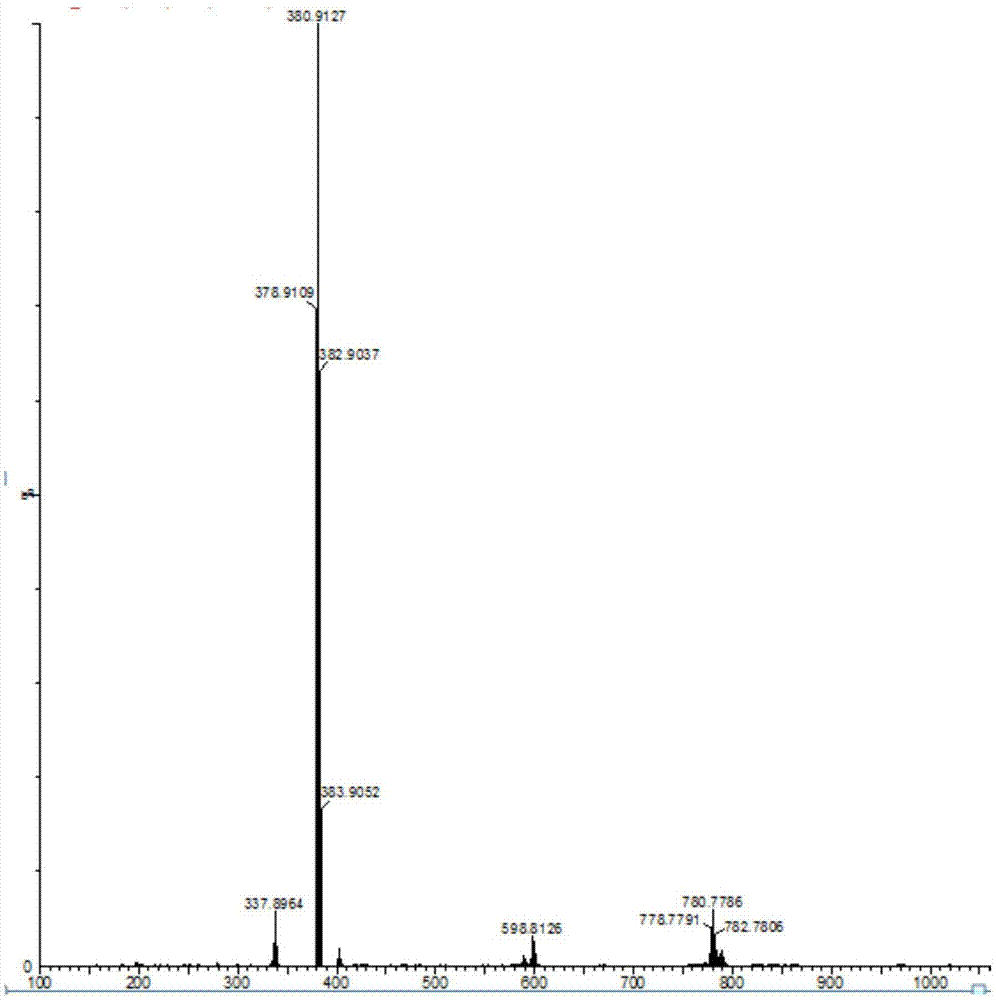
[0040] Dissolve 5.0 mL of 2-bromophenylacetyl chloride in 40 mL of tetrahydrofuran, and slowly drop it into 0.85 mL of 80% hydrazine hydrate solution in 0.85 mL of tetrahydrofuran (20 mL) through a constant-pressure low-liquid funnel at 0° C., and react for 1 hour. Then react at room temperature for 2 hours, wash with saturated sodium bicarbonate solution until alkaline, filter with suction to obtain a white solid, wash with water, and dry to obtain 2,5-bis(2-bromophenyl)-1,3,4-oxo Oxadiazole product, (yield: 91%), LC-MS (EI) m / z 380.9127 [M + ].
[0041] The second step: Synthesis of 2-spiro-9,9′-xanthene fluorenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborane
[0042] Add 2-bromo-spiro-9,9′-xanthene fluorene (0.4110g, 1.0mmol) and 50mL tetrahydrofuran into a single-necked round bottom flask, stir at low temperature ...
Embodiment 2
[0045] Example 2: Synthesis of 2,5-bis(2-(spiro-9,9'-thiaxanthene fluorene)phenyl)-1,3,4--oxadiazole
[0046] The first and second steps are the same as the first and second steps of Example 1,
[0047] The third step: 2,5-bis(2-bromophenyl)-1,3,4-oxadiazole (0.3800g, 1.0mmol), 2-spiro-9,9′-thioxanthene fluorenyl -4,4,5,5-tetramethyl-1,3,2-dioxaborane (0.9480g, 2.0mmol), tetrakistriphenylphosphopalladium (0.2311g, 0.2mmol), potassium carbonate solution (10.0 mL, 2.0mol / L) and toluene into a three-necked round-bottomed flask, heated and stirred for 5-72 hours under nitrogen protection and light-proof conditions, and then cooled to room temperature after the reaction was evaporated and concentrated by a rotary evaporator, washed with water, Dichloromethane was extracted several times, and the organic phase was combined and dried with anhydrous magnesium sulfate; Crude product; then column chromatography, using ethyl acetate and petroleum ether as detergents, after purification...
Embodiment 3
[0048] Example 3: Synthesis of 2,5-bis(2-(spiro-9,9'-dioxathioxanthene fluorene)phenyl)-1,3,4--oxadiazole
[0049] The first and second steps are the same as the first and second steps of Example 1,
[0050] The third step: 2,5-bis(2-bromophenyl)-1,3,4-oxadiazole (0.3800g, 1.0mmol), 2-spiro-9,9′-dioxathioxanthene Fluorenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborane (1.0120g, 2.0mmol), tetrakistriphenylphosphopalladium (0.2311g, 0.2mmol), potassium carbonate solution (10.0mL, 2.0mol / L) and toluene were added to a three-necked round-bottomed flask, under nitrogen protection and light-shielding conditions, heated and stirred for 5-72 hours, and then the reaction was cooled to room temperature, evaporated and concentrated by a rotary evaporator, Washing with water and extracting with dichloromethane several times, combining the organic phases and drying with anhydrous magnesium sulfate; after drying, suction filtration under reduced pressure and washing the desiccant with dichlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com