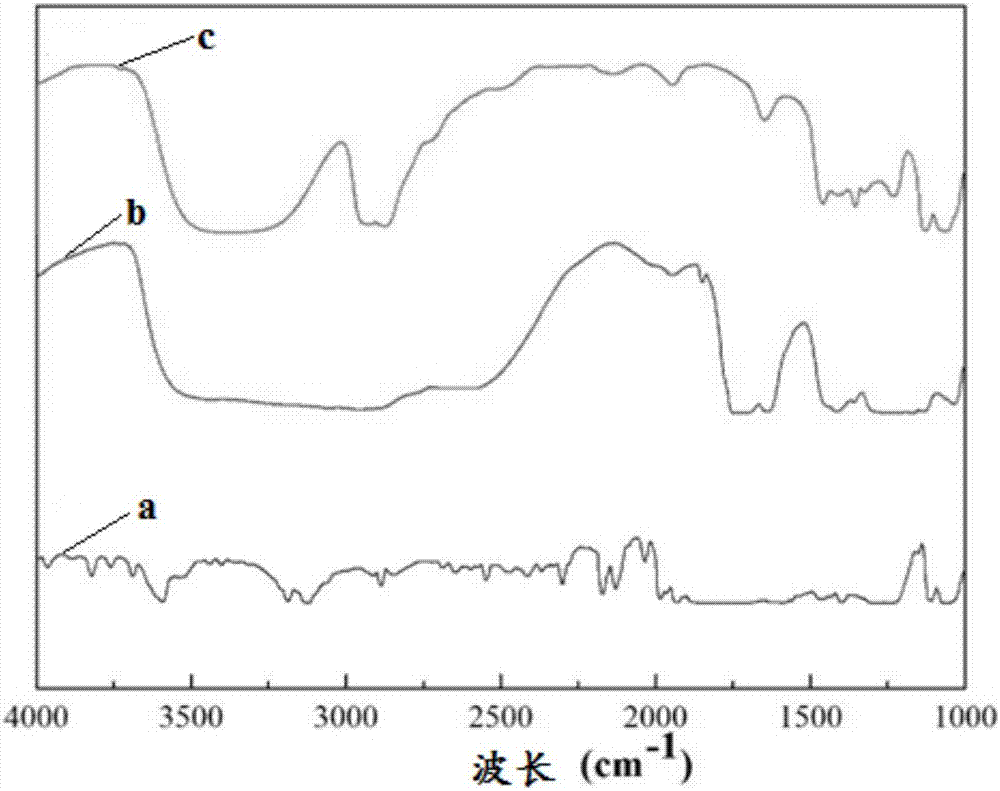
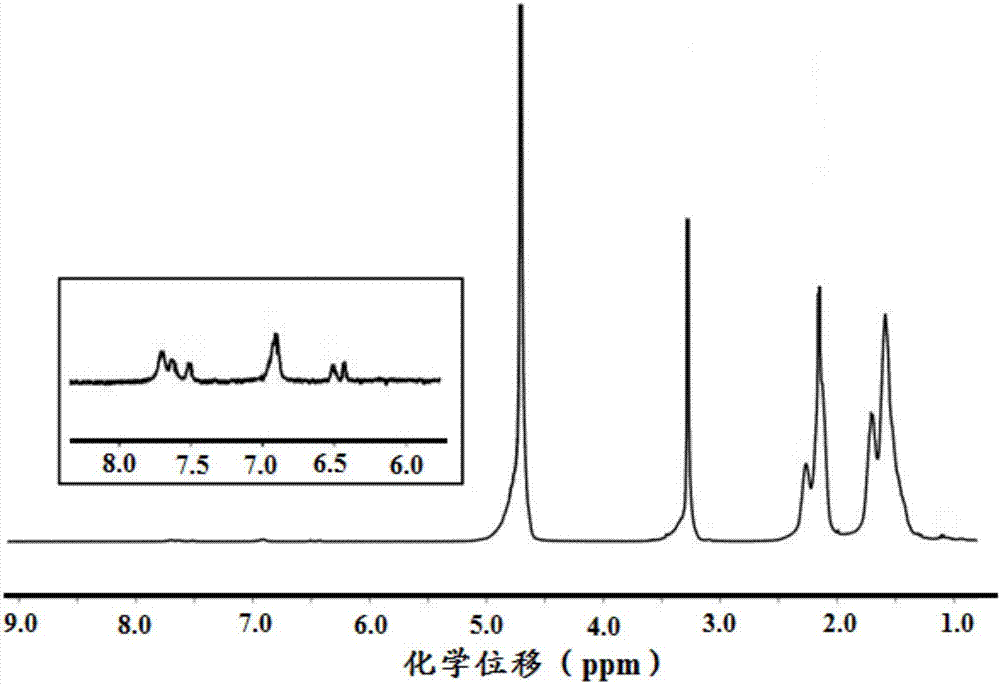
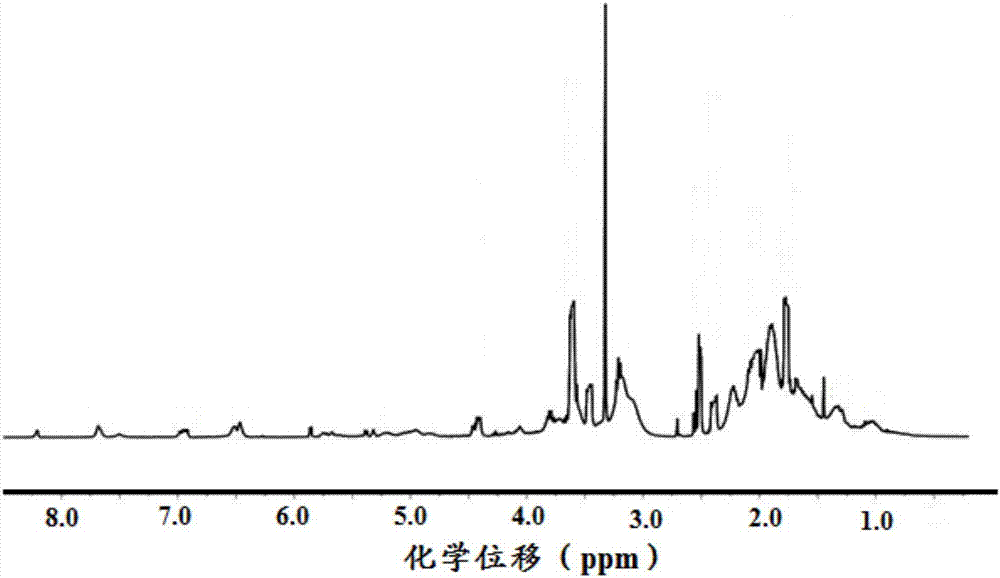
In-situ cross-linked hydrogel
An in-situ cross-linking and hydrogel technology, applied in the field of hydrogel, can solve the problems of low reaction efficiency, complex preparation of dienophiles, and large gaps in hydrogel cross-linking structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0043] According to a preferred embodiment of the present invention, the furan-based monomer is a monomer containing a furan group and a polymerizable double bond.
[0044] In a further preferred embodiment, the furyl monomer is selected from one or more of furfuryl methacrylate, furyl acrylic acid, and furyl acrylonitrile.
[0045] In a further preferred embodiment, the furyl monomer is selected from furfuryl methacrylate and / or furyl acrylic acid.
[0046] Wherein, the water-soluble monomer and the furan-based monomer are copolymerized to obtain the polymer main agent, and the main active site of the polymer main agent is the furan side group produced after the furan-based monomer is polymerized, wherein, the water-soluble monomer is added to carry out The purpose of copolymerization is as follows: first, to reduce the steric hindrance effect of furan-based monomers and improve the polymerization efficiency of the polymer main agent; second, to control the cross-linking dens...
Embodiment 1
[0156] (1) Preparation of polymer main agent
[0157] Weigh 99g of acrylamide, 1g of furfuryl methacrylate, and 900g of butanone as a solvent, and add them to a reactor equipped with a reflux condenser. After stirring to dissolve the monomers, fill with nitrogen and exhaust oxygen for 30 minutes, heat up to 65°C, and add 0.5g Initiator azobisisobutyronitrile, stirred and reacted at constant temperature for 6 hours, added 0.5 g of chain terminator hydroquinone, stirred for 20 minutes, stopped the reaction, cooled and discharged, filtered to obtain polymer filter cake, and dried under reduced pressure to obtain polymer main agent .
[0158] (2) Preparation of crosslinking agent
[0159] Take maleic anhydride 33.0g (0.336mol), (diethylene glycol) 17.0g (0.160mol), add in the reactor that reflux condenser is housed, add 50g (0.861mol) acetone and stir to dissolve reactant, Then add 0.25 g (0.003 mol) of catalyst anhydrous sodium acetate, pass through nitrogen to remove the air, ...
Embodiment 2
[0163] (1) Preparation of polymer main agent
[0164] Weigh 100g of hydroxyethyl acrylate, 4.5g of furyl acrylic acid, and 900g of butanone as a solvent, add them into a reactor equipped with a reflux condenser, stir to dissolve the monomers, fill with nitrogen and exhaust oxygen for 30min, heat up to 75°C, add 0.5 g initiator benzoyl peroxide, after stirring and reacting at constant temperature for 8 hours, add 0.5 g of chain terminator methyl hydroquinone, stop the reaction after stirring for 20 minutes, cool and discharge, filter to obtain polymer filter cake, dry under reduced pressure to obtain polymer main agent.
[0165] (2) Preparation of crosslinking agent
[0166] Take maleic anhydride 31.9g (0.325mol), butanediol 14.7g (0.163mol), add in the reactor that reflux condenser is housed, add 50g (0.861mol) acetone stirring and dissolving reactant, then add 0.5g ( 0.006mol) catalyst anhydrous sodium acetate, pass nitrogen to remove the air, heat up to 50°C, stir at const...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Compressive strength | aaaaa | aaaaa |
| Compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com