Fluorescence immunochromatographic assay test paper for kanamycin residues and preparation method of fluorescence immunochromatographic assay test paper
A technique of fluorescence immunochromatography and kanamycin is applied in the field of fluorescence immunochromatography test paper for detecting kanamycin and its preparation, which can solve the problems of insufficient quantitative detection and sensitivity, low cost and the like, and achieve obvious economic benefits and advantages. Social benefits, improved sensitivity, and the effect of ensuring food safety and protecting consumers' health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: prepare the kanamycin antibody conjugate (Eu 3+ -Kana-ab)
[0047] (1) 200 mg of dextran was dissolved in 5 ml of 0.03 M sodium periodate solution, protected from light, and reacted overnight at room temperature. Then, it was dialyzed overnight with ultrapure water to remove unreacted small molecules, and stored at 4°C for future use.
[0048] (2) The kanamycin antibody was taken and dialyzed overnight with 25mM pH 8.0 carbonic acid buffer solution (CBS for short).
[0049] (3) Take the prepared Eu 3+ -BHHCT@SiO 2 4 mg of fluorescent nanoparticles were suspended in 400 μL of 25 mM carbonate buffer solution with pH 9.6, and then 400 μL of aldylated dextran was added, and reacted in the dark for 7 hours. Wash 3 times with 25mM carbonate buffer of pH 9.6, and suspend in 200 μL of 25mM carbonate buffer of pH 9.6.
[0050] (4) Mix 200 μL of the dialyzed Kanna antibody with the above-mentioned aldehyddextran-modified fluorescent nanoparticles, and react ove...
Embodiment 2
[0052] Example 2: Preparation of Kanamycin Residual Fluorescent Immunoassay Test Strips
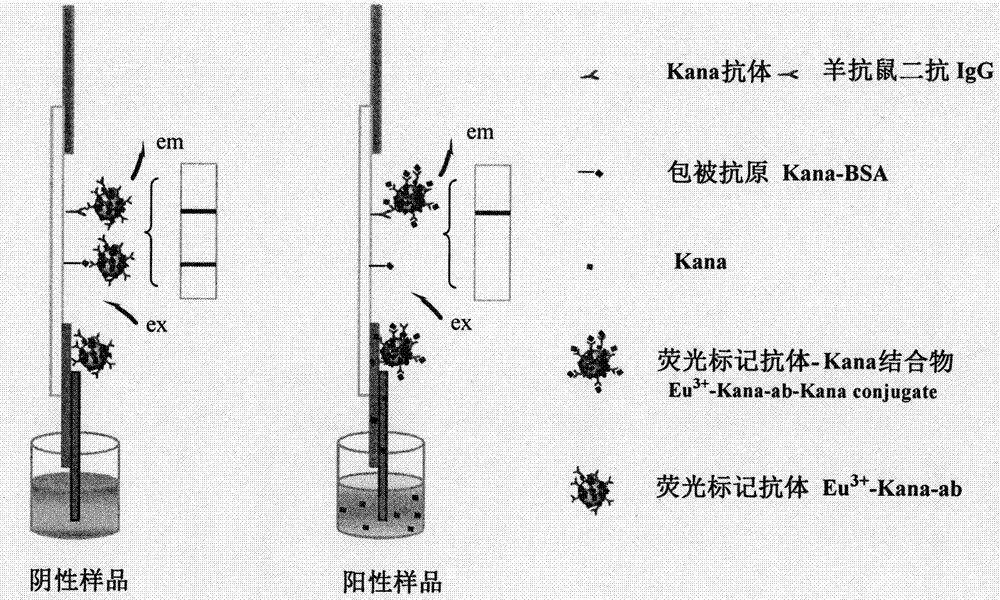
[0053] (1) Immobilization of fluorescently labeled antibodies: Dilute the prepared fluorescently labeled antibodies with 200mM pH 7.8 Tris-HCl buffer at a certain ratio; , and then freeze-dried for later use.
[0054] (2) Coating of quality control line (C line) and detection line (T line): on the NC membrane, use a dot film device to spot the coated antigen Kana-BSA into a strip as the detection line (T line); On the 0.5cm membrane of the detection line, draw a control line with goat anti-mouse secondary antibody IgG as the quality control line (C line), and dry it for later use.
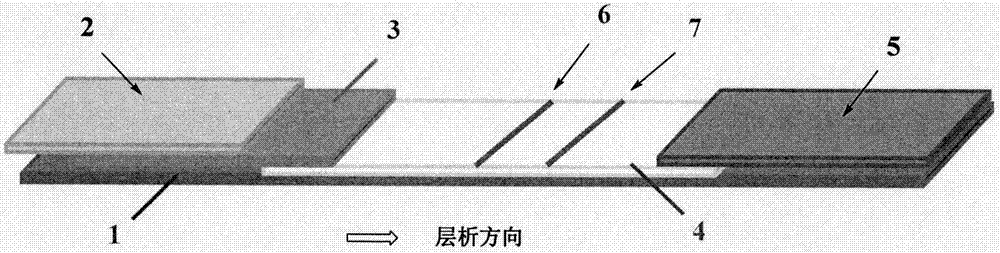
[0055] (3) Assembly of test strips: paste NC film on the middle of a rectangular bottom plate, mark the line with a film spotter, paste glass fiber on one end of the film, and lay an antibody-binding pad on the transfer side of the film. Glue absorbent paper to the other end. Then cut into 4mm strip test st...
Embodiment 3
[0058] Example 3: Preparation of Kanamycin Residual Fluorescent Immunoassay Strips, Different Fluorescently Labeled Antibodies Eu 3+ -Research experiment on the effect of Kana-ab dilution factor on the fluorescence performance of test strips
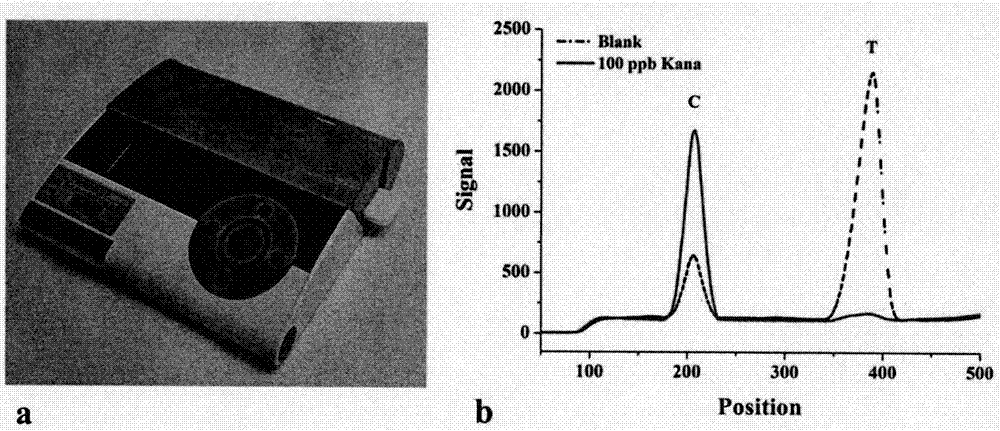
[0059] Antibodies labeled with fluorescent rare earth silica nanoparticles (Eu 3+ -Kana-ab) solution was diluted 1:1000, 1:1500, 1:2000, 1:4000, added 150 μL of blank sample, reacted in the dark, and carried out gel electrophoresis imaging detection and fluorescence card reader measurement.
[0060] Gel electrophoresis ( Figure 4 a) shows that, with Eu 3+ - As the dilution ratio of Kana-ab increases, the brightness of C-line and T-line gradually becomes lighter. When the dilution ratio is 1000 times, 1500 times and 2000 times, there is little difference in brightness visually. In the process of making fluorescent test strips, the ratio of the fluorescence intensity of the T line to the C line is close to 1, which is the best, such as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com