Rana rugulosa wiegmann antibacterial peptide, as well as gene and application thereof
An antimicrobial peptide and tiger frog technology, applied in the field of biomedicine, can solve the problems of insufficient research on skin active peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, tiger frog antimicrobial peptide gene cloning:
[0028] 1, tiger frog skin total RNA extraction: the living tiger frog is cleaned with water, put into liquid nitrogen and quick-frozen for 4h, get skin tissue, weigh, get 300mg skin tissue, add 10m total RNA extraction buffer (Trizol solution, the U.S. GIBCOBRL company product), homogenized in 20m1 glass homogenizer for 30min. Add an equal volume of phenol / chloroform solution, mix vigorously, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, and discard the precipitate. Add an equal volume of isopropanol to the supernatant, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, wash the precipitate once with 75% ethanol, and dry it. The precipitate at the bottom of the tube is the total RNA of tiger frog skin.
[0029] II. Purification of tiger frog skin mRNA: the separation and purification of tiger frog skin mRNA was carried out by...
Embodiment 2
[0041] Embodiment 2, the preparation of tiger frog antimicrobial peptide:
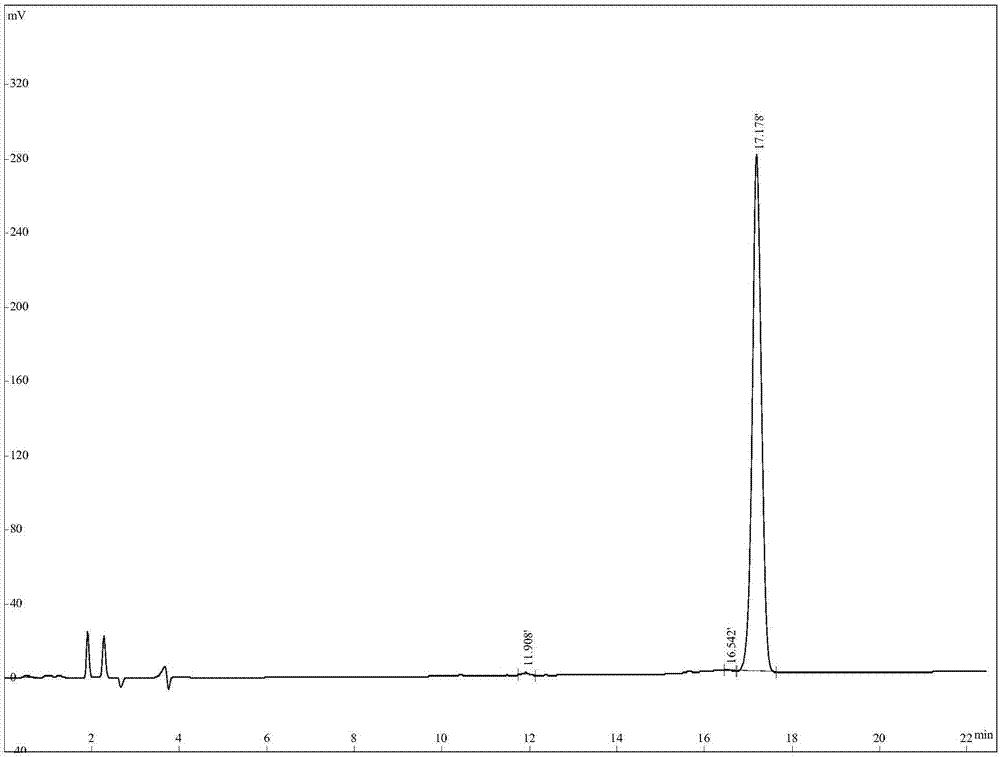
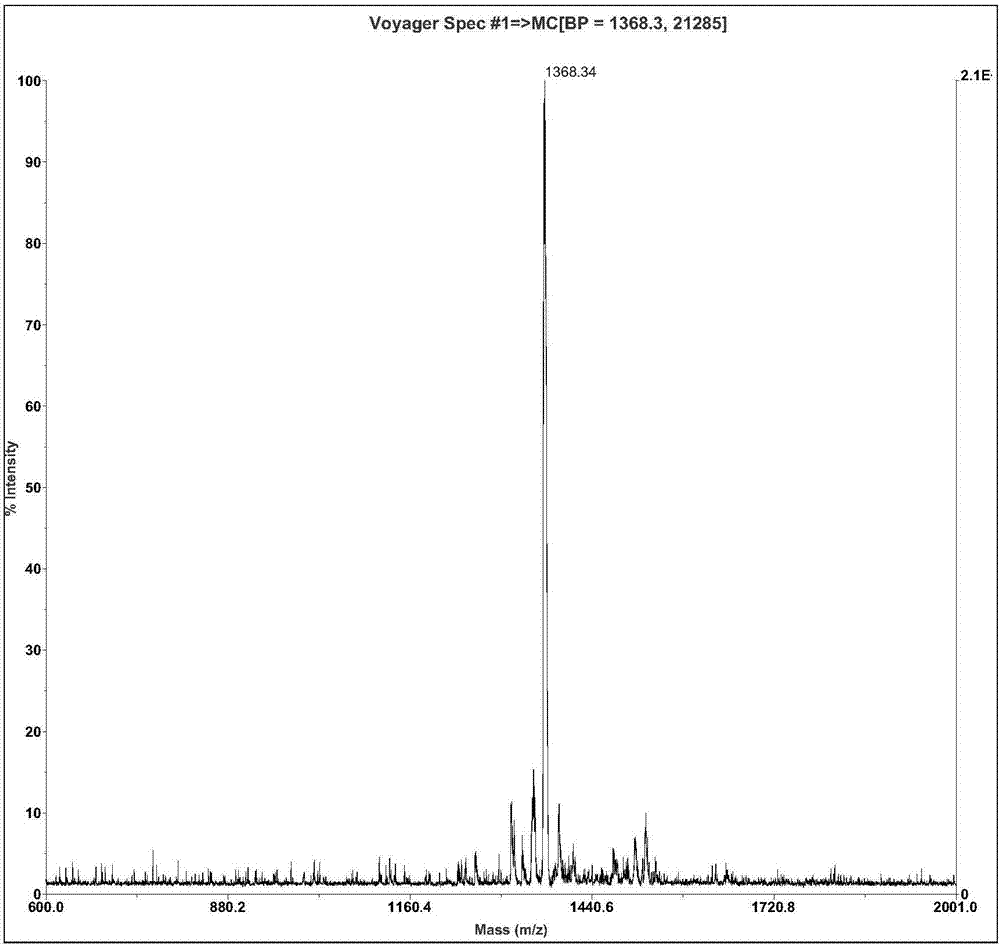
[0042] Ⅰ. The preparation method of the antibacterial peptide of tiger frog: according to the gene of tiger frog antibacterial peptide, the amino acid sequence of the mature active antimicrobial peptide encoded by the function is deduced, and then the polypeptide is synthesized by an automatic polypeptide synthesizer. The formation of disulfide bond adopts the air oxidation method, specifically dissolving the polypeptide in the flask according to 0.1mg / ml in 0.1% acetic acid solution, titrating with ammonium hydroxide to pH 7.8, and then stirring overnight at room temperature. Desalted and purified by HPLC reverse phase C18 column chromatography. Liquid A is 0.05% TFA+2% CH during purification 3 CN, liquid B is 0.05% TFA+90% CH 3 CN, the gradient of peptide elution is that the concentration of solution B is 25-47% within 20 minutes, the detection wavelength is 220nm, and the peptide appears at 11.908...
Embodiment 3
[0046] Embodiment 3, activity test of tiger frog antimicrobial peptide
[0047] Ⅰ. Determination of the ability to inhibit bacterial growth
[0048]The antibacterial activity was detected by the cup and saucer method, and the medium was ordinary agar medium. Inject 20m1 of heated and melted culture medium into the plate as the bottom layer, spread it evenly in the bottom of the plate, after solidification, take another appropriate amount of culture medium and heat it to melt, then add 5m1 of bacterial suspension to each plate, shake well, Spread it evenly on the bottom layer as a bacterial layer. After cooling, put 6 sterilized stainless steel cups evenly in the plate at equal distances. Add 0.1 ml of the test compound solution at a concentration of 0.1-0.3 mg / ml to the first steel cup, add the sample solution to the remaining steel cups by doubling the dilution method, incubate at 37°C, and observe the size of the inhibition zone. The bacteriostatic zone above 10mm was reg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com