Method for synthesis of atazanavir
A synthetic method and compound technology, applied in the field of preparation of protease inhibitors, can solve the problems of many synthetic steps and high production cost, and achieve the effects of simplified synthetic steps, simple operation and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
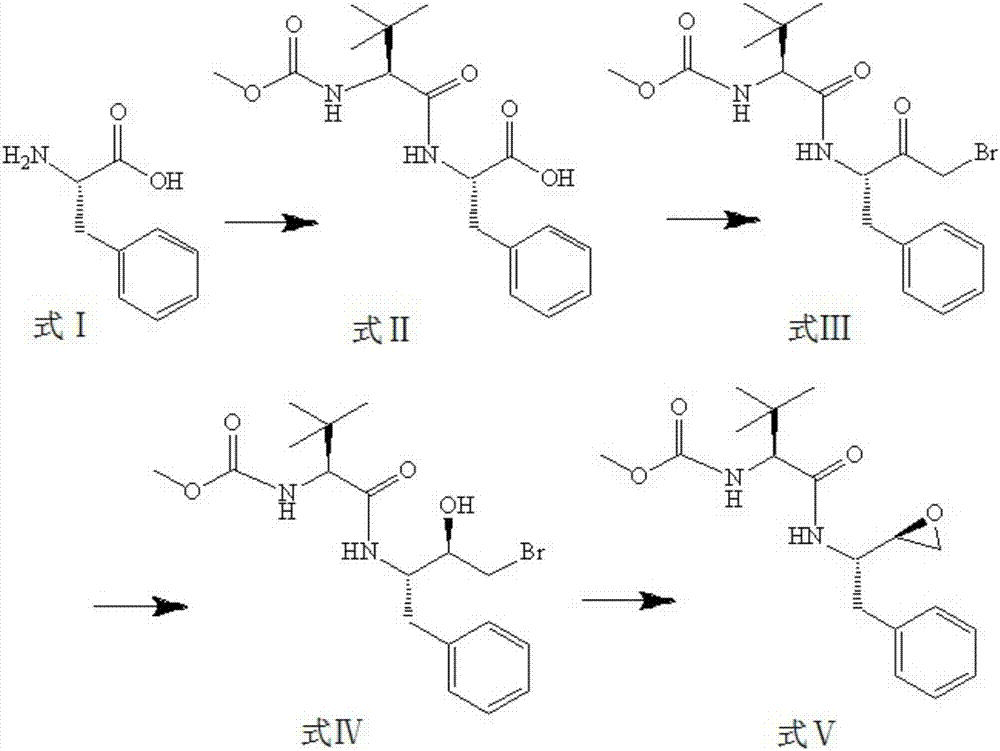
[0038]Formula V compound (S)-1-((S)-2-oxiranyl-1-phenylethane-2-yl-amino)-3,3-dimethyl-1-carbonylbutane-2 The synthetic method of -base-carbamate methyl ester comprises the steps:
[0039] 1) Formula I compound L-phenylalanine (74.6g, 451.9mmol) reacts with N-methoxycarbonyl-L-tert-leucine (90.8g, 480.0mmol) in 1000ml of dichloromethane, using a condensing agent of (150 g, 0.5 mol) of 3-(diethoxyphosphoryloxy)-1,2,3-benzotriazin-4-one DEPBT and (50 g, 0.26 mol) of 1-(3-dimethyl Aminopropyl)-3-ethylcarbodiimide DECI mixture, add 20ml triethylamine, and react at 0-20°C for 6-10h to obtain formula II compound (S)-1-((S )-phenylpropanoic acid-2-yl-amino)-3,3-dimethyl-1-carbonylbutan-2-yl-carbamate methyl ester (138.6 g, 412.1 mmol);
[0040] 2) Add 138.6g of the compound of formula II to a mixed solvent of tetrahydrofuran and ether, first add isobutyl chloroformate and react in ice bath for 20min, then add diazomethane for 3h, then add hydrogen bromide and react for 40min at -20...
Embodiment 2
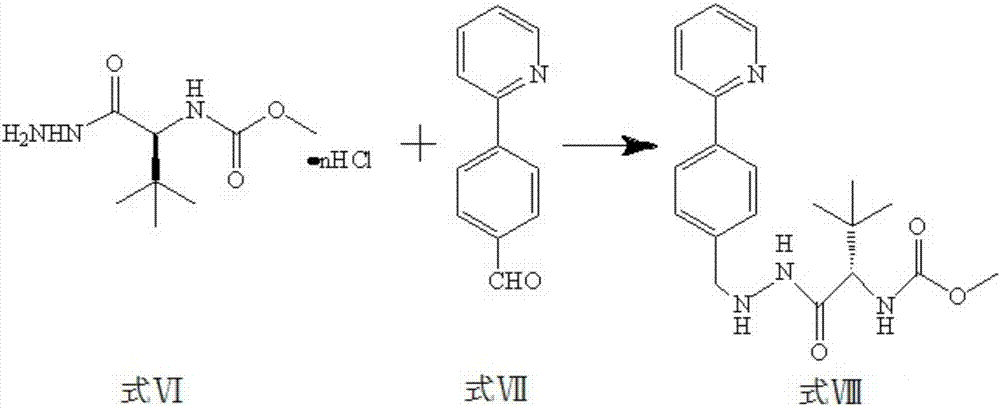
[0046] Under nitrogen protection, triethylamine (21.9 mL, 158.5mmol), stirred at room temperature for 1 hour, added a solution of ethanol (250ml) dissolved in the compound of formula VII 4-(2-pyridyl)-benzaldehyde (65.1g, 355.6mmol), refluxed at 45°C for 5h, and cooled to room temperature , adding LiAlH 4 (8g, 0.25mol), hydrogenated at normal pressure for 12 hours. Filtration, the filtrate was concentrated, and the resulting crude product was recrystallized with isopropanol to obtain formula VIII compound N-1-[N-(methoxycarbonyl)-L-tert-leucine base]-N-2-[4-( 2-pyridyl)-benzyl]hydrazine (122.2g, 330.0mmol);
[0047] The reaction formula is as follows:
[0048]
Embodiment 3
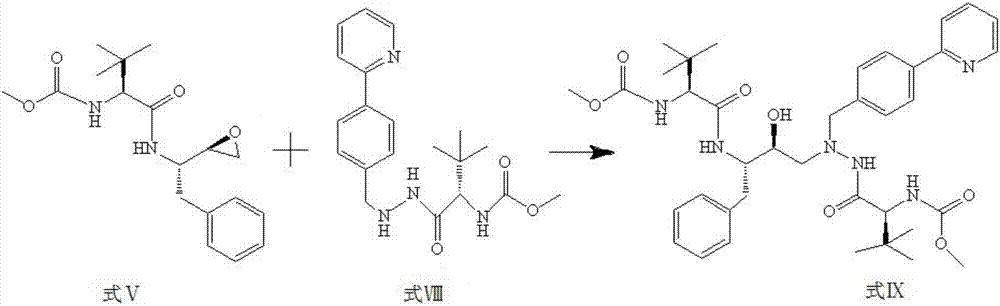
[0050] (102.0g, 305.0mmol) Formula V compound (S)-1-((S)-2-oxiranyl-1-phenylethane-2-yl-amino)-3,3-dimethyl- 1-Carbonylbutane-2-yl-methyl carbamate and (122.2g, 330.0mmol) compound of formula VIII N-1-[N-(methoxycarbonyl)-L-tert-leucine base]-N-2 -[4-(2-Pyridyl)-benzyl]hydrazine was dissolved in 500ml of isopropanol, refluxed at 90°C for 12h, cooled, slowly added 1000ml of distilled water, stirred for 2h, left standing, filtered, washed with water, and then washed with ethanol Water recrystallization, vacuum drying, liquid phase detection, obtain 201.3g formula IX compound 1-[4-(2-pyridyl)phenyl]-5(S)-2,5-bis{[N-(methoxycarbonyl )-L-tert-leucine base]amino}-4(S)-hydroxyl-6-phenyl-2-azahexaneⅧ, which is atazanavir.
[0051] The reaction formula is as follows:
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com