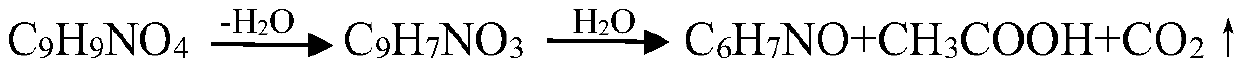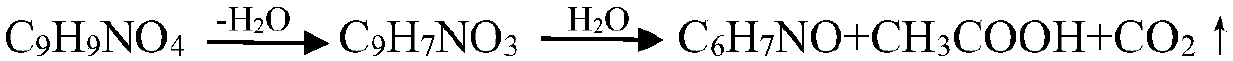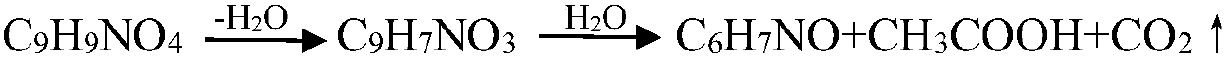Technological method for preparing m-aminophenol
A m-aminophenol and aminophenol technology, applied in the field of m-aminophenol preparation, can solve the problems of low yield and selectivity, high equipment requirements, serious pollution, etc., and achieve easy-to-obtain raw materials, high product yield, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of processing method for preparing m-aminophenol in the present embodiment is carried out successively according to the following steps:
[0033] 1, the preparation of m-cresyl acetate of m-aminophenol reaction raw material:
[0034] A. The reaction equation is as follows:
[0035] C 7 h 8 O+CH 3 CClO→C 9 h 10 o 2 +HCl↑
[0036] B, the preparation steps are:
[0037] First, take m-cresol 21.6g in a 100ml Erlenmeyer flask, then add 15ml of dichloromethane as a solvent to the Erlenmeyer flask; secondly, under 1°C ice-water bath condition, use a constant pressure dropping funnel to dissolve 15.7g of acetyl chloride reagent Add it dropwise into the system, and fully react for 30 minutes; again, continue to react at a low temperature in an ice-water bath at 1°C for 60 minutes, then start to heat up, control the temperature of the system at 55°C, and react for 2 hours. The tail gas generated by the reaction is passed into an aqueous solution of sodium hydroxide...
Embodiment 2
[0056] A kind of processing method for preparing m-aminophenol, carries out successively according to the following steps:
[0057] 1, the preparation of m-cresyl acetate of m-aminophenol reaction raw material:
[0058] A. The reaction equation is as follows:
[0059] C 7 h 8 O+CH 3 CClO→C 9 h 10 o 2 +HCl↑
[0060] B, the preparation steps are:
[0061] First, take m-cresol 21.6g in a 100ml Erlenmeyer flask, then add 15ml of dichloromethane as a solvent to the Erlenmeyer flask; secondly, under 3°C ice-water bath conditions, use a constant pressure dropping funnel to dissolve 15.7g of acetyl chloride reagent Add it dropwise into the system, and fully react for 45 minutes; again, continue to react at a low temperature in an ice-water bath at 3°C for 60 minutes, then start to heat up, control the temperature of the system at 60°C, and react for 2.5 hours. At last, the solid mixture generated after the reaction in the Erlenmeyer flask is taken out, and the unreacted sol...
Embodiment 3
[0080] A kind of processing method for preparing m-aminophenol, carries out successively according to the following steps:
[0081] 1, the preparation of m-cresyl acetate of m-aminophenol reaction raw material:
[0082] A. The reaction equation is as follows:
[0083] C 7 h 8 O+CH 3 CClO→C 9 h 10 o 2 +HCl↑
[0084] B, the preparation steps are:
[0085] First, take m-cresol 21.6g in a 100ml Erlenmeyer flask, and then add 15ml of dichloromethane as a solvent to the Erlenmeyer flask; secondly, under 5°C ice-water bath conditions, use a constant pressure dropping funnel to dissolve 15.7g of acetyl chloride reagent Add it dropwise into the system, and fully react for 60 minutes; again, continue to react at a low temperature of 5°C in an ice-water bath for 60 minutes, then start to heat up, control the temperature of the system at 65°C, and react for 3 hours. The tail gas generated by the reaction is passed into the sodium hydroxide aqueous solution At last, the solid mixt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com