Glycosyl substituted genipin derivative as well as preparation method and application thereof
A technology of genipin and derivatives, which is applied to a class of glycosyl-substituted genipin derivatives and their preparation and application fields, to achieve excellent insecticidal activity and excellent bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
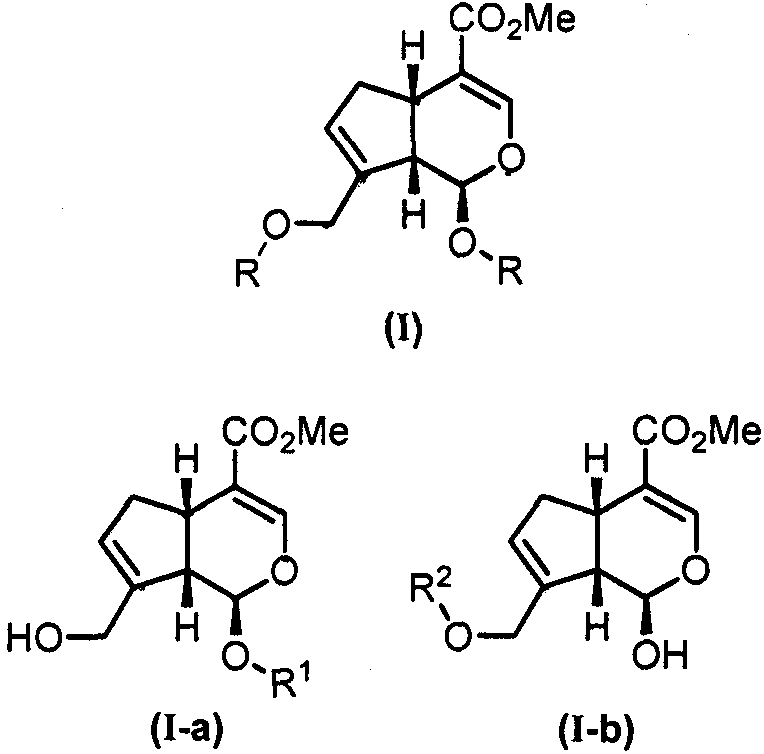
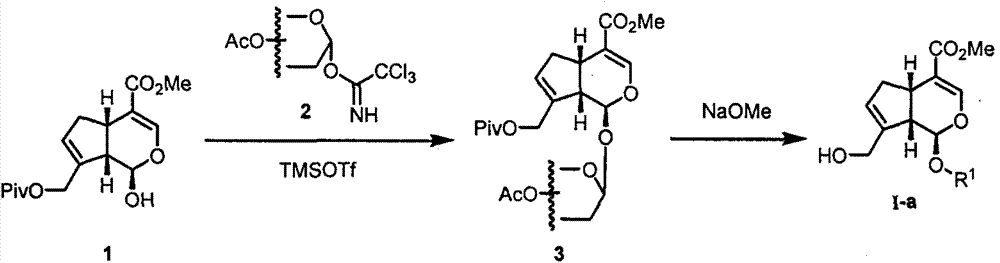
[0026] Embodiment 1: Synthesis of I-a-1~I-a-9
[0027] Synthesis of 1-hydroxygenipin (1): Add genipin (500mg, 2.21mmol), pyridine (0.267mL, 3.315mmol) and dichloromethane (22mL) into a 100mL round bottom flask. Under the protection of argon, the reaction temperature was lowered to 0° C., and pivaloyl chloride (0.299 mL, 2.431 mmol) was added dropwise. After the addition was complete, the reaction was heated up and stirred overnight. After the reaction was completed, saturated ammonium chloride was added to the organic phase to quench the reaction, and the organic phase was washed with 5% copper sulfate aqueous solution and saturated sodium chloride aqueous solution, and dried over anhydrous sodium sulfate. After concentration under reduced pressure, separation by column chromatography (petroleum ether: ethyl acetate = 5:1) gave 551 mg of a gray solid, with a yield of 80%.
[0028] Target compound (I-a-1): Add glycosyl trichloroacetimidate (2, 1mmol), 1-hydroxygenipin (1, 1mmo...
Embodiment 2
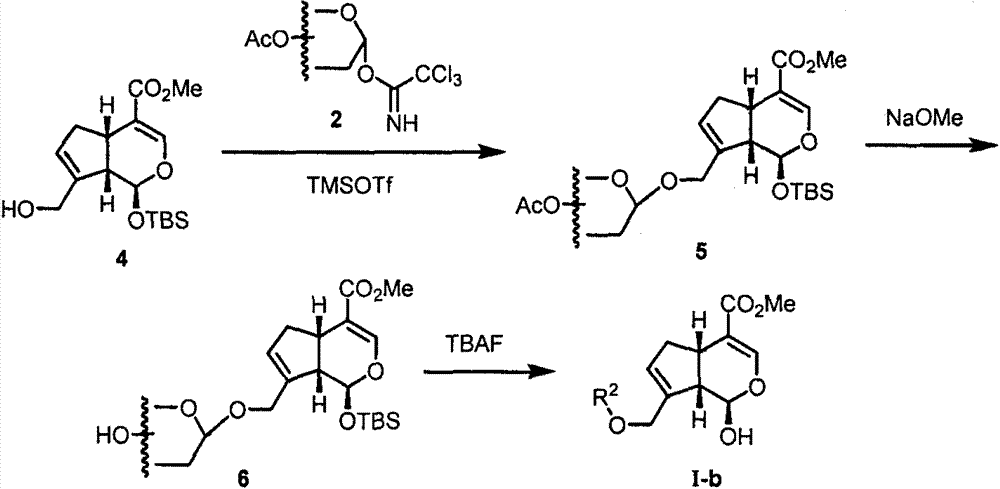
[0041] Embodiment 2: Synthesis of I-b-1~I-b-9
[0042] Synthesis of 7-hydroxygenipin (4): Add genipin (1.13g, 5mmol), silver nitrate (2.12g, 12.5mmol) and 30mL solvent N,N-dimethylformamide in a 100mL round bottom flask , tert-butyldimethylsilyl chloride (1.88 g, 12.5 mmol) was slowly added with stirring at 0° C., and the reaction was stirred overnight at room temperature. After the reaction was completed, the reaction system was filtered, the filtrate was poured into saturated aqueous sodium bicarbonate solution, and extracted three times with ether. The organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. Precipitation by filtration, column chromatography (petroleum ether: ethyl acetate = 40:1-5:1) yielded 334.5 mg colorless oil of monosubstituted product and 1.5 g colorless oil of double substituted product, total yield 86% . In a 100 mL round bottom flask, mono-substituted genipin (1.49 g, 3.28 mmol) was dissolved in 20 mL ...
Embodiment 3
[0057] Embodiment 3: the assay of anti-TMV activity, assay procedure is as follows:
[0058] 1. Virus purification and concentration determination:
[0059] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0060] 2. Compound solution preparation:
[0061] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL stock solution, and then diluted to the required concentration with aqueous solution containing 1‰ Tween 80.
[0062] 3. In vitro therapeutic effect:
[0063] Rub inoculation of leaves of Shanxi tobacco at the right age, rinse with running water, the virus concentration is 10 μg / mL. After drying, cut it off, cut it in half along ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com