Preparation method of halcinonide and derivative thereof
A technology of derivatives and products, which is applied in the field of preparation of halcinide and its derivatives, can solve the problems of difficult control of catalytic reactions, poor yields, side reactions, etc., so as to inhibit the generation of side reaction products and improve the effect of catalysis , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
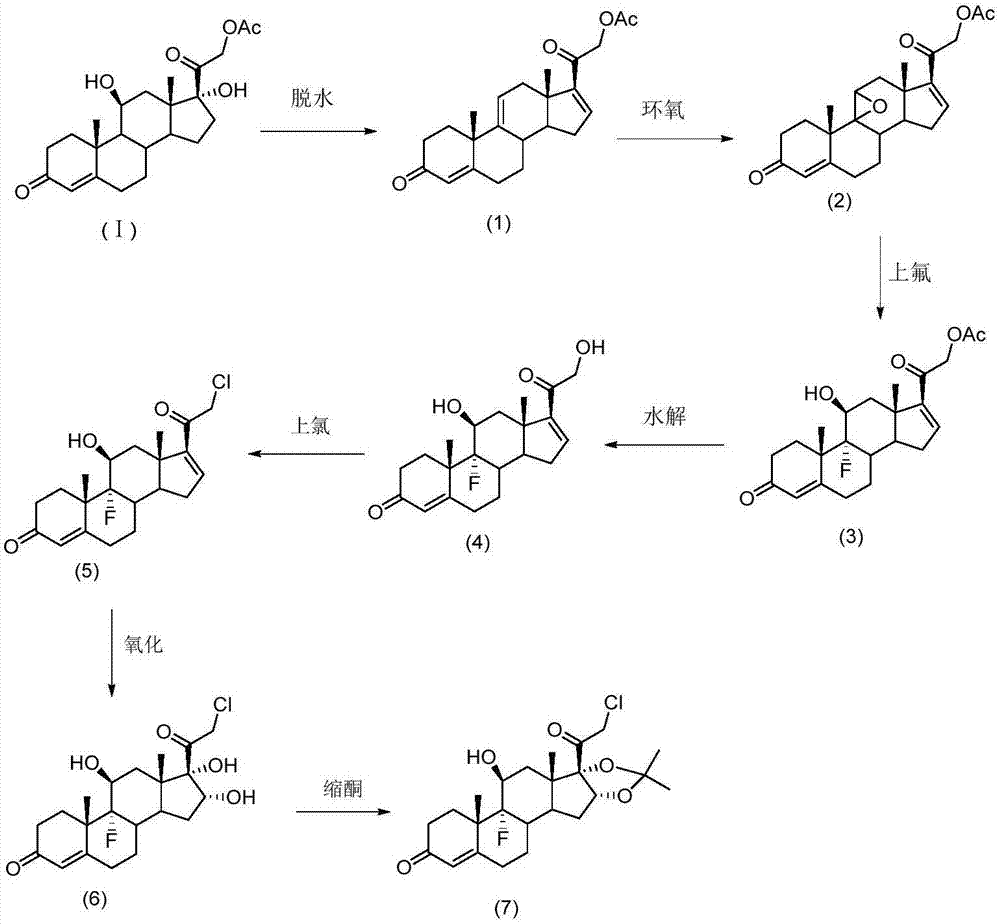
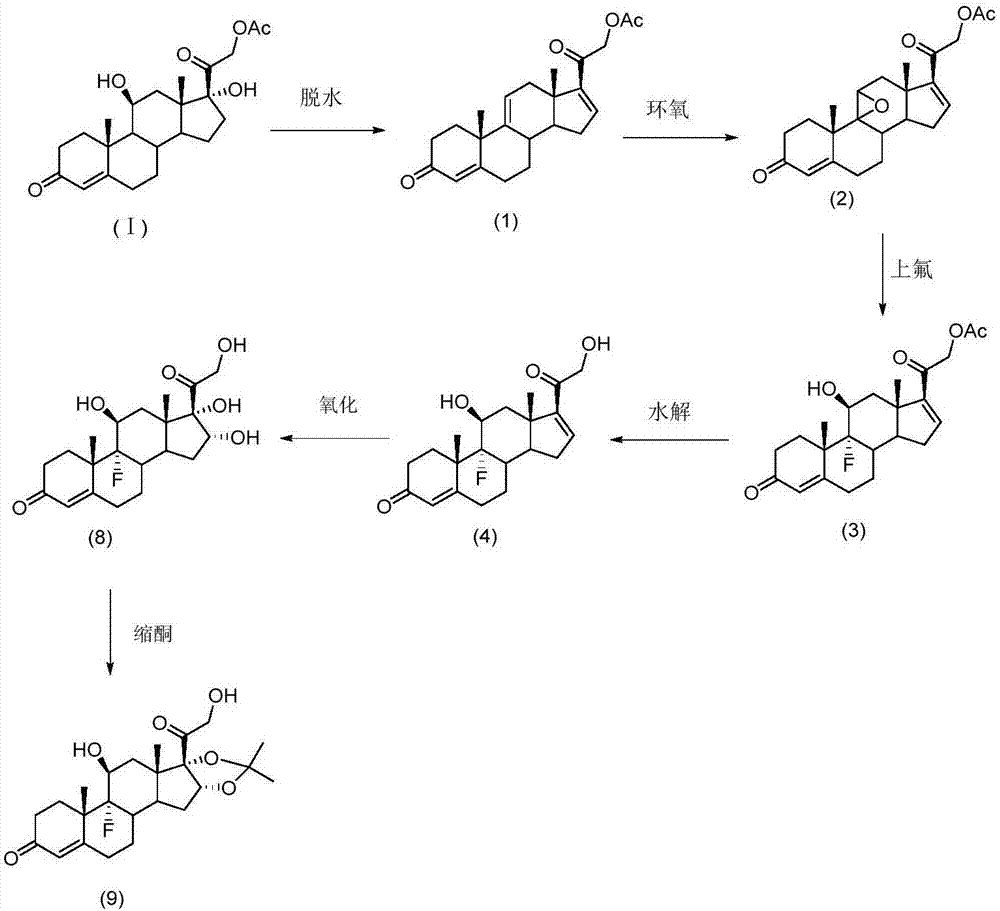
[0112] Prepare Halcinonide as follows:
[0113] (a) After dissolving hydrocortisone acetate in N,N-dimethylformamide, controlling the temperature of the solution at about -20°C, and then adding concentrated sulfuric acid for dehydration to obtain the dehydration intermediate (1);
[0114] (b) dissolving the dehydration intermediate (1) in tetrahydrofuran, controlling the temperature of the solution at about 0°C, and then adding dibromocyanoacetamide and perchloric acid to obtain the halide intermediate;
[0115] Then, the temperature of the solution is raised and controlled at about 30° C., and sodium hydroxide solution is added to obtain the epoxy intermediate (2);
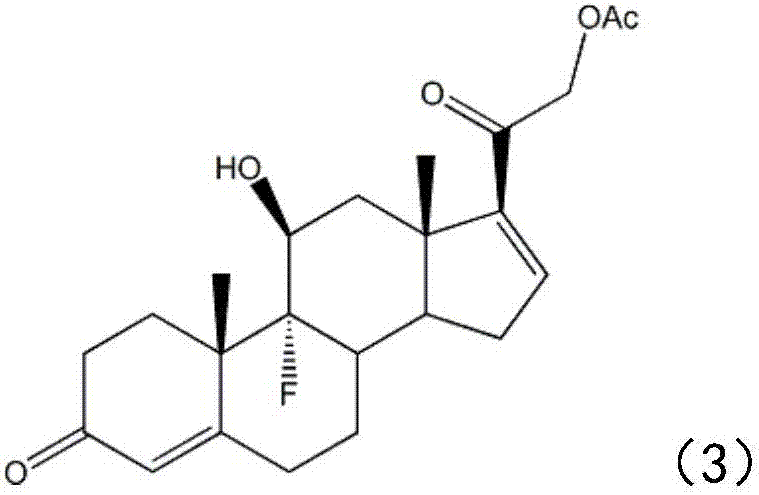
[0116] (c) adding the epoxy intermediate (2) into the dimethylformamide solution, then adjusting the temperature of the solution to about -10°C, adding a HF / DMF solution with a concentration of 35%, and performing ring-opening and fluorination reactions, The ring-opening intermediate (3) is obtained;
[0117] (...
Embodiment 2
[0123] Prepare Halcinonide as follows:
[0124] (a) After dissolving hydrocortisone acetate in N,N-dimethylacetamide, the temperature of the solution is controlled at about 0°C, and then phosphorus pentachloride is added for dehydration to obtain the dehydration intermediate (1);
[0125] (b) dissolving the dehydration intermediate (1) in acetone, controlling the temperature of the solution at about 10°C, and then adding N-bromosuccinimide and nitric acid to obtain the halide intermediate;
[0126] Then, after the halide intermediate is separated, it is dissolved in the acetone solution again, and the temperature of the solution is controlled at about 50° C., and potassium hydroxide solution is added to obtain the epoxy intermediate (2);
[0127] (c) Add the epoxy intermediate (2) into the tetrahydrofuran solution, then adjust the temperature of the solution to about -40°C, add a HF / DMF solution with a concentration of 45%, and perform ring-opening and fluorination reactions t...
Embodiment 3
[0134] Prepare hydrotriamcinolone acetonide according to the following steps:
[0135] (a) After dissolving hydrocortisone acetate in N,N-dimethylformamide, controlling the temperature of the solution at about -20°C, and then adding concentrated sulfuric acid for dehydration to obtain the dehydration intermediate (1);
[0136] (b) dissolving the dehydration intermediate (1) in tetrahydrofuran, controlling the temperature of the solution at about 0°C, and then adding dibromocyanoacetamide and perchloric acid to obtain the halide intermediate;
[0137] Then, the temperature of the solution is raised and controlled at about 30° C., and sodium hydroxide solution is added to obtain the epoxy intermediate (2);
[0138] (c) adding the epoxy intermediate (2) into the dimethylformamide solution, then adjusting the temperature of the solution to about -10°C, adding a HF / DMF solution with a concentration of 35%, and performing ring-opening and fluorination reactions, The ring-opening in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com