Preparation method of bisphenol A hapten and bisphenol A complete antigen
A complete antigen and hapten technology, applied in the preparation of organic compounds, carboxylate salts, chemical instruments and methods, etc., can solve the cumbersome preparation of bisphenol A hapten and complete antigen, low product purity, complicated operation, etc. problems, to achieve the effect of short synthesis time, high product purity and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1 1
[0062] Experimental example 1 Sodium monochloroacetate Na 2 CO 3 Methods Synthesis of Bisphenol A Hapten
[0063] Weigh 640 mg of sodium monochloroacetate powder and dissolve it in 4 mL of water to obtain solution A; weigh 270 mg of anhydrous sodium carbonate and dissolve it in 2 mL of water to obtain solution B; weigh 1140 mg of bisphenol A particles and dissolve it in 40 mL of dioxane to obtain Solution C: first add solution A dropwise to solution C, then add solution B dropwise to the mixed solution of A and C, and stir for 9 hours in a water bath at 70°C. Remove the insoluble matter by filtration, evaporate the filtrate under reduced pressure to remove the solvent, suspend the remaining residue in 10mL distilled water, adjust the pH to 3.6 with 6mol / L HCl solution, filter the precipitate, dissolve it in an appropriate amount of boiling chloroform, and add a small amount of petroleum ether , a white precipitate was formed, which was filtered and dried.
[0064] The UV sc...
experiment example 2
[0065] Experimental example 2 Ethyl bromobutyrate K 2 CO 3 (Method 1) Synthesis of bisphenol A hapten
[0066] Weigh 1.14g (5mmol) BPA and 828mg (6mmol) anhydrous K 2 CO 3 Dissolve in 20 mL of anhydrous acetone, add 716 μL of ethyl bromobutyrate, heat the mixture to 70 ° C, and stir under reflux for 8 h. The reaction was cooled and dried by rotary evaporation under vacuum. Mix 10 mL of methanol and 5 mL of 10% NaOH solution, dissolve the solid residue after rotary evaporation and drying in the above mixed solution, and reflux for 30 min under heating. Adjust to pH=2 with 1:1 HCl solution. Add appropriate amount of ethyl acetate to extract 3 times, collect the ethyl acetate phase, anhydrous Na 2 SO 4 After dehydration, the solvent was removed by rotary evaporation.
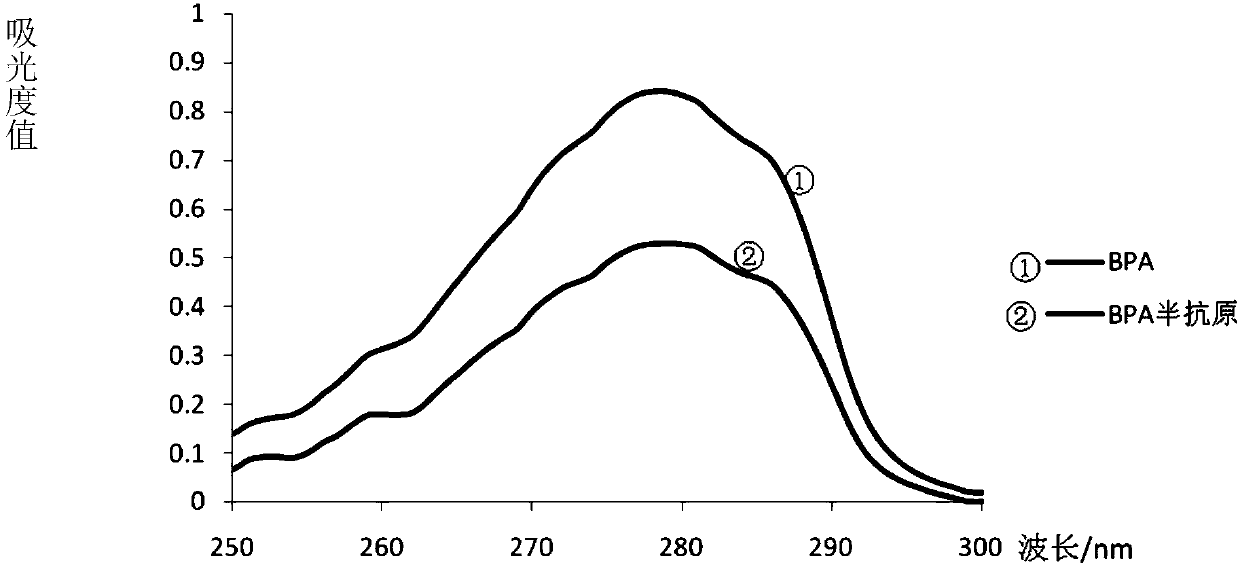
[0067] The UV scan results of BPA standard (100μg / mL) and hapten (100μg / mL) figure 2 . Comparing the two curves, it can be seen that both the BPA standard and the BPA hapten have characteristic absorptio...
experiment example 3
[0069] Experimental example 3 Ethyl bromobutyrate K 2 CO 3 (Method 2) Synthesis of bisphenol A hapten
[0070] Weigh 1.004g (4.4mmol) BPA and 1.348g (9.75mmol) anhydrous K 2 CO 3 Dissolve in 10 mL of anhydrous DMF to obtain a mixed solution, add 697 μL (4.87 mmol) ethyl bromobutyrate dropwise to the above mixed solution, and stir overnight at 75° C. under airtight condition. Add 5 mL of 10% NaOH solution, heat for 30 min, and adjust to pH=2 with 1:1 HCl solution. The mixed solution was extracted 3 times with ethyl acetate, 30mL each time, the ethyl acetate phase was collected, and anhydrous Na 2 SO 4 Dehydration treatment, evaporated to dryness under reduced pressure.
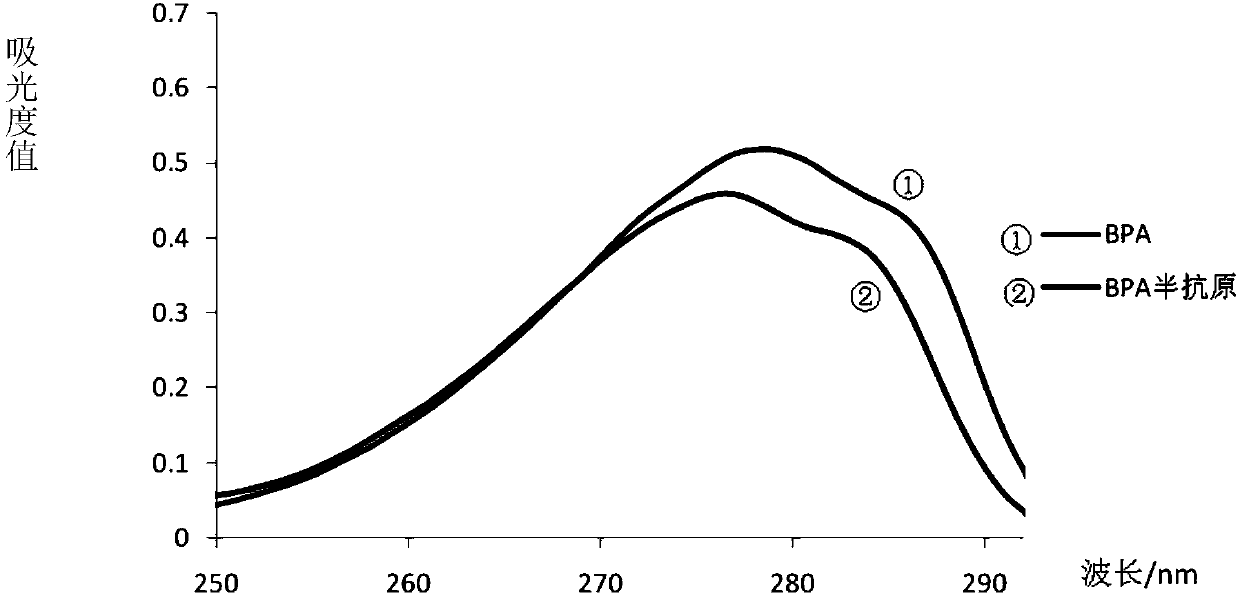
[0071] The UV scan results of BPA standard (100μg / mL), DPA standard (100μg / mL) and BPA hapten (100μg / mL) Figure 4 . Comparing the three curves, it can be seen that the BPA standard, DPA standard and BPA hapten all have characteristic absorption peaks at 278nm, and there is no obvious shift in the maxim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com