Fluorine atom substituted benzo heterocycle based conjugated molecule material as well as preparation method and application thereof
A technology of conjugated molecules and fluorine atoms, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of organic solar cell energy conversion rate attenuation, high price, weak absorption, etc., and achieve the improvement of carrier Transport characteristics, good planarity and rigidity, and the effect of promoting charge transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
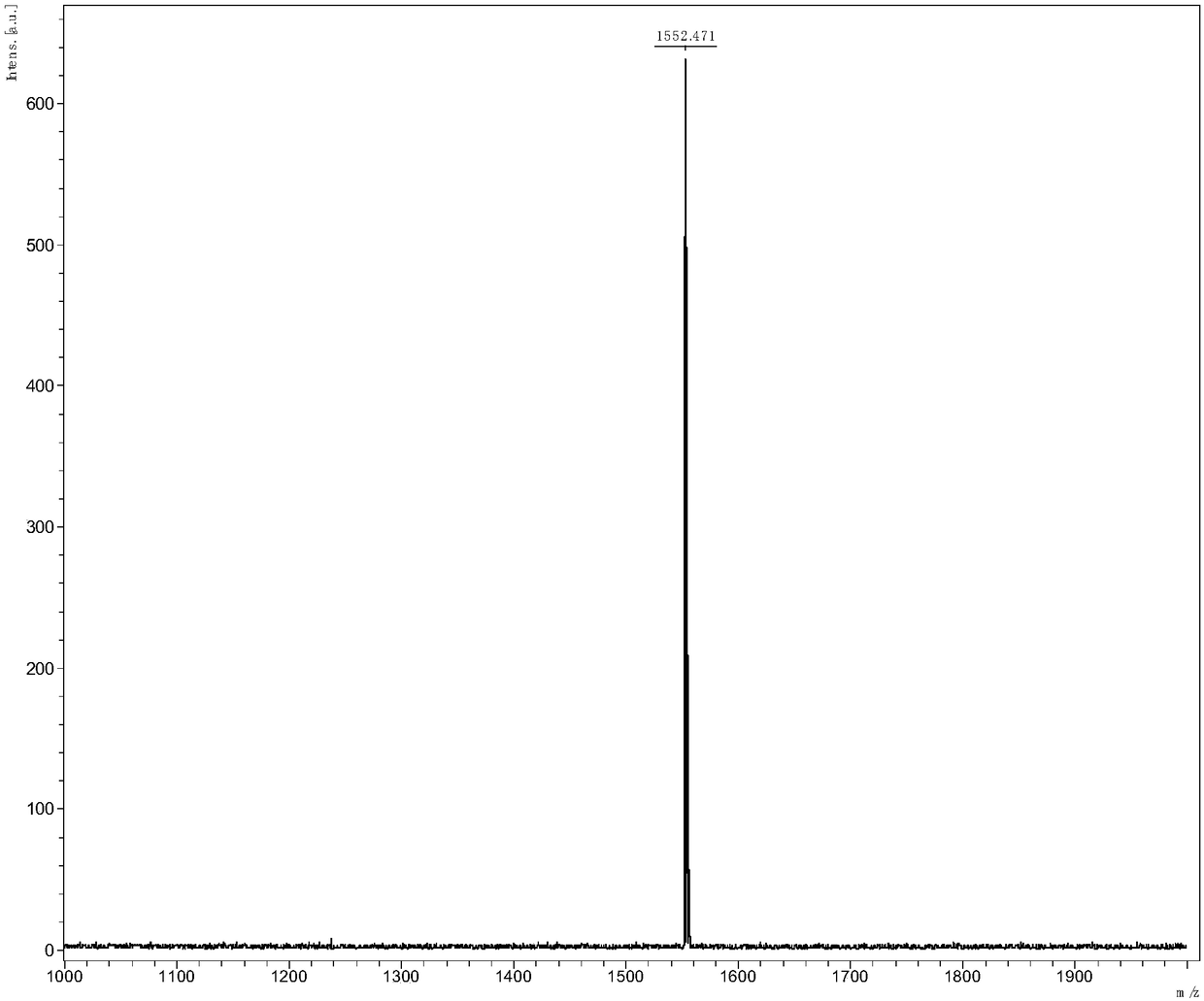
[0060] Synthesis of Conjugated Molecular Materials (IffBR) Based on Indadodithiophene, 5,6-Fluorobenzo[d][1,2,5]thiadiazole and Rhodamine
[0061] The synthetic route is as follows:
[0062]
[0063] Preparation of 4-Bromo-5,6-difluoro-7-methylbenzene[c][1,2,5]thiadiazole (Compound 1)
[0064] Under argon atmosphere, in a 100 mL two-necked round bottom flask, dissolve 4-bromo-5,6-difluorobenzene[c][1,2,5]thiadiazole (1.0 g, 4.0 mmol) in 50 mL of tetrahydrofuran , lithium diisopropylamide (12 mL, 2.0 Min THF) was added dropwise and stirred at -78 °C for 1 h. Iodomethane (1.2 g, 8.0 mmol) was then added to the reaction mixture, the reaction apparatus was returned to room temperature, and the reaction was stirred for 8 hours. After the reaction was completed, the reaction was quenched with water, poured into water, extracted with dichloromethane, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The crude product was further p...
Embodiment 2
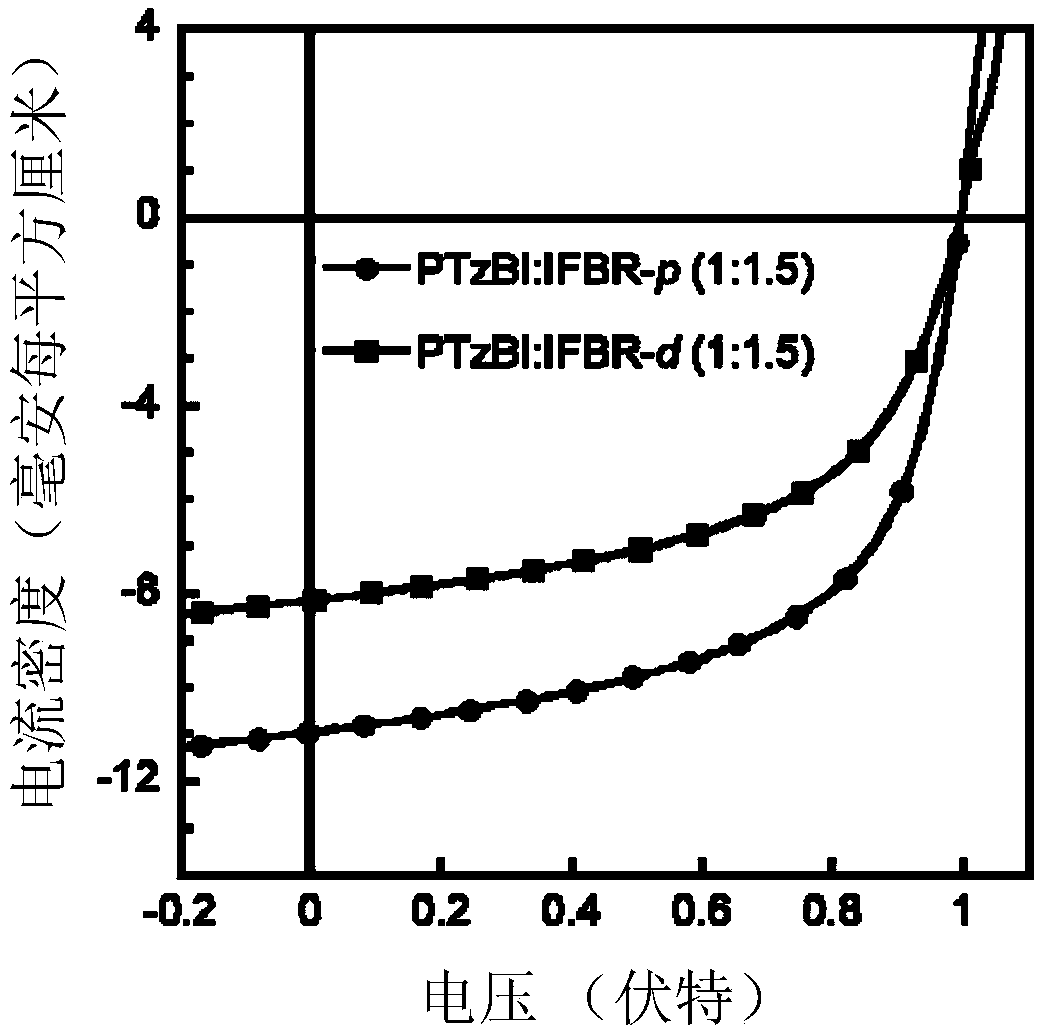
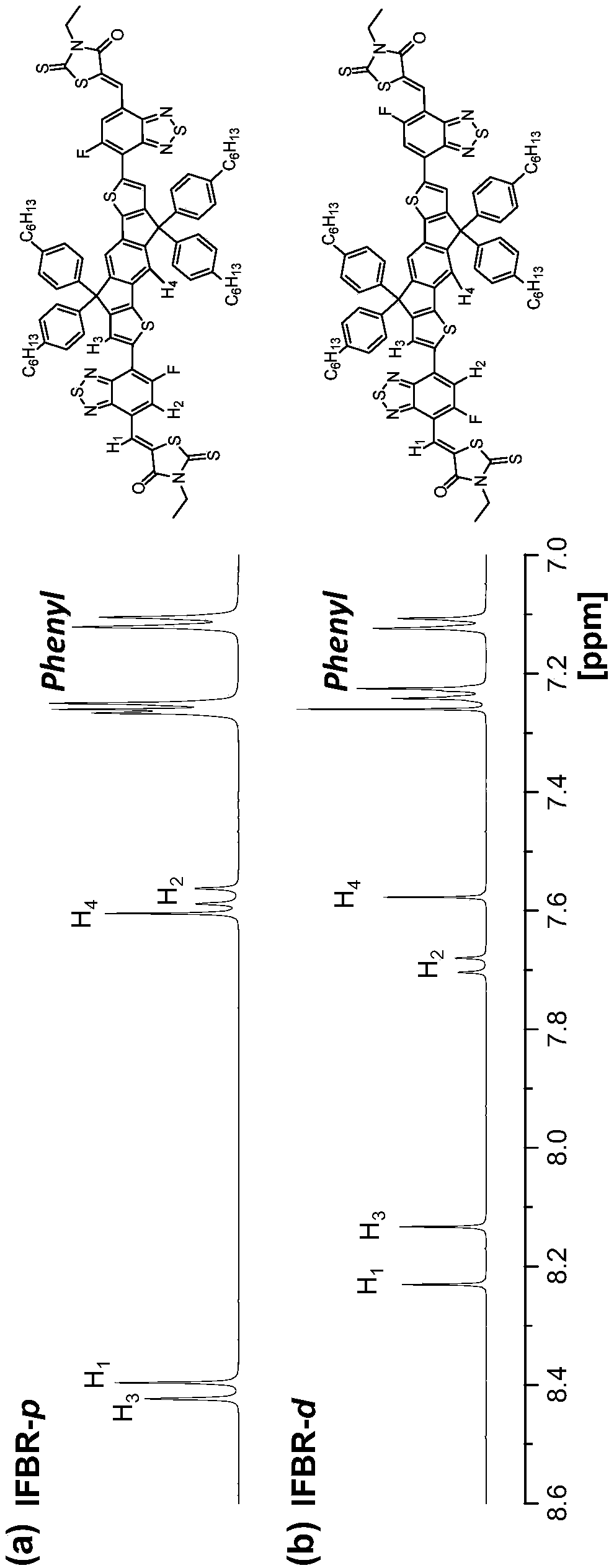
[0075] Synthesis of Conjugated Molecular Materials (IFBR-p for short) based on indahenodithiophene, 5-fluorobenzo[d][1,2,5]thiadiazole and rhodamine
[0076] The synthetic route is as follows:
[0077]
[0078] Preparation of 5-Fluoro-3-methylbenzene-1,2-diamine (Compound 6)
[0079] Under an argon atmosphere, in a 250 mL two-necked round-bottomed flask equipped with a reflux condenser, add 5-fluoro-3-methyl-2-nitroaniline (5.0 g, 29.4 mmol) and dissolve it in 50 mL of ethanol , while adding Fe powder (8.2 g, 147 mmol), then the reaction device was placed in an oil bath, and the temperature was raised to 80 °C. Then HCl (31 mL, 12M) diluted with 30 mL of ethanol was added slowly. The reaction was refluxed for 8 hours and the temperature was lowered to room temperature. The reaction solution was poured into cold NaOH aqueous solution, extracted with ethyl acetate, and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 3...
Embodiment 3
[0095] Synthesis of Conjugated Molecular Materials (IFBR-d) Based on Indabodithiophene, 5-Fluorobenzo[d][1,2,5]thiadiazole and Rhodamine
[0096] The synthetic route is as follows:
[0097]
[0098] Preparation of 2-bromo-4-fluoro-6-nitroaniline (compound 12)
[0099] Under an argon atmosphere, in a 250 mL two-necked round-bottomed flask equipped with a condensing device, 4-fluoro-2-nitroaniline (5 g, 32 mmol) was dissolved in 35 mL of acetic acid, and the liquid Br was added dropwise. 2 (10.24 g, 64 mmol) was added to the reaction apparatus. The reaction apparatus was then placed in an oil bath, heated to 60°C and held for 8 hours. After the reaction was complete, the reaction mixture was then poured into NaHSO 3 In aqueous solution, 7 g of yellow solid were obtained. The product did not require further purification. Yield 93%.
[0100] Preparation of 3-bromo-5-fluorobenzene-1,2-diamine (compound 13)
[0101] Under an argon atmosphere, in a 250 mL two-necked round-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com