Method for producing phenyllactic acid through whole-cell transformation of phenylalanine
A technology of phenylalanine and phenyllactic acid, which is applied in the field of enzyme engineering, can solve the problems of being unsuitable for industrial production and the high price of phenylpyruvate, and achieve a high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Cloning of Example 1 L-amino acid deaminase mutant D165K / F263M / L336M gene
[0030] The l-aad gene was cloned using the laboratory-preserved plasmid PET20b-(l-aad) (D165K / F263M / L336M) as a template. The PCR product was digested with BamHI and HindIII, and further inserted into the MCS-1 site of the vector pRSF-Duet-1 to construct the recombinant plasmid pRSF-aad.
Embodiment 2
[0031] Example 2 Collinearity Analysis and Systems Biology Selection of Lactate Dehydrogenase
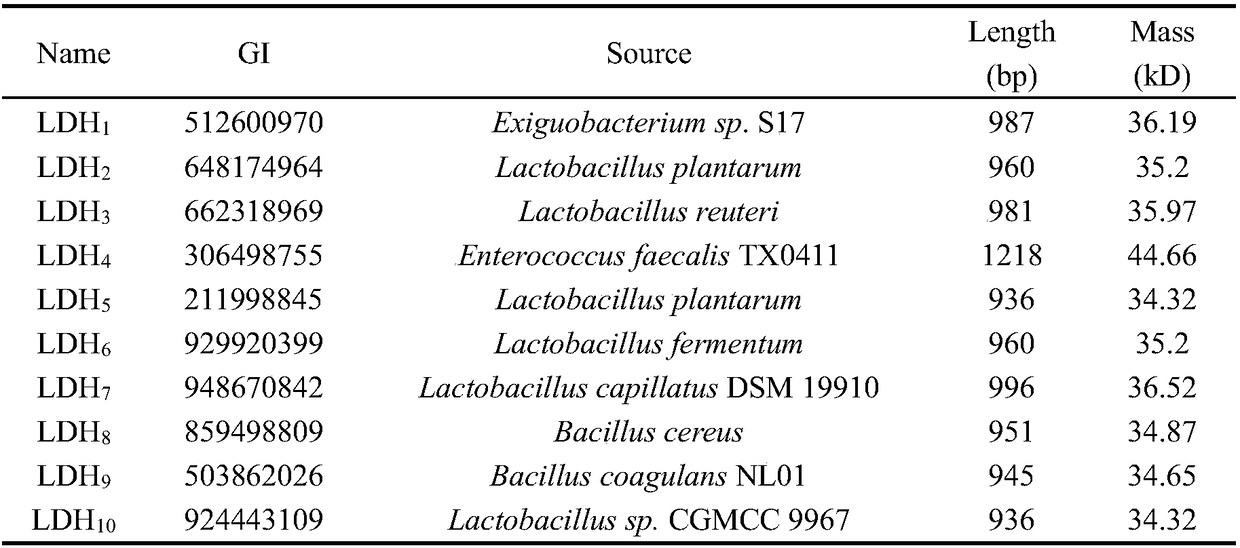
[0032] The known genes with higher lactate dehydrogenase activity were BcLDH from Bacillus coagulans NL01, L-nLDH and D-nLDHfrom from Bacillus coagulans SDM, and LaLDH from Lactobacillus sp.SK007. Non-redundant protein databases in GenBank were searched for homologous sequences using BLASTP 2.2.28+. Use H-CD-HIT (CD-HIT suite) to group the retrieval results, there are three levels: 90%, 60%, 40% similarity. Using the NCBI taxonomy database, rebuild taxonomy trees. After performing PSI-BLAST on the sequences of the four enzymes, the sequence PSI-BLAST results were obtained, and Synteny was analyzed using GCView. Finally, ten different sources of lactate dehydrogenase genes with certain sequence differences were selected for the next experiment.
[0033] Table 1. Lactate dehydrogenase from different sources
[0034]
Embodiment 3
[0035] Example 3 Construction of L-amino acid deaminase gene and L-lactate dehydrogenase gene co-expression plasmid
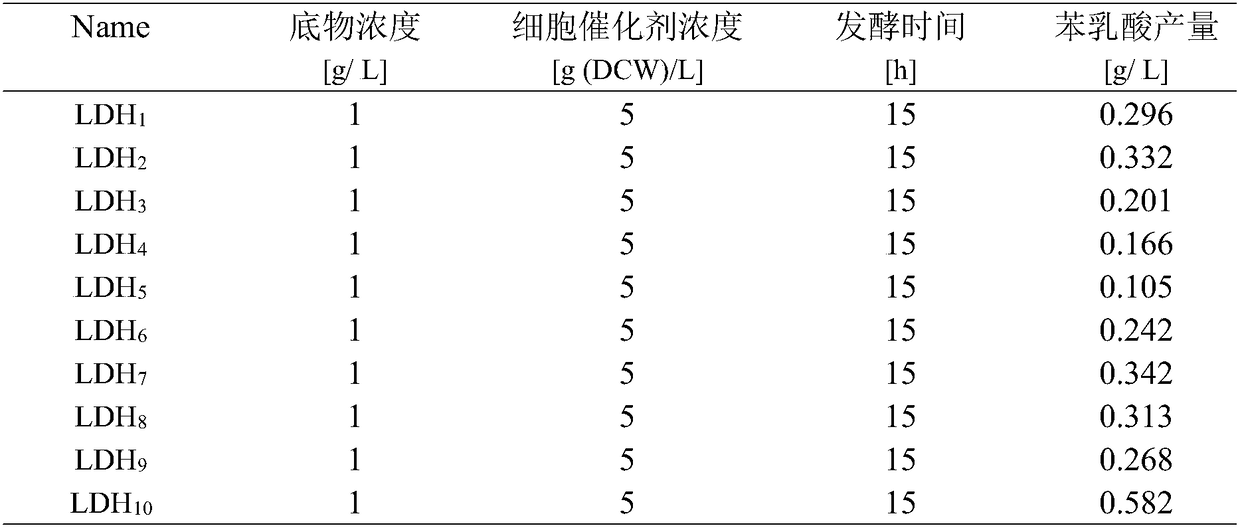
[0036] After screening 10 ldh genes according to Example 2, the codons were optimized according to the gene sequence in the gene bank. Then, it was synthesized by Reddy Biotechnology Co., Ltd. (Shanghai, China), and two restriction sites NdeI and XhoI were added in the forward and reverse directions, respectively. The ldh gene was amplified by PCR. The resulting product was inserted into the plasmid pRSF-aad MS2 site, and the resulting recombinant plasmid was named pRSF-aad-ldh, and further transformed into Escherichia coli BL21(DE3). The obtained 10 recombinant strains were added with 0.4 mM IPTG to induce expression at 25° C. for 10 h. After the induction, the fermentation broth was centrifuged to collect the cells, and the cells were resuspended with phenylpyruvate as the substrate for whole-cell transformation, and the optimal ldh gene was screened out ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com