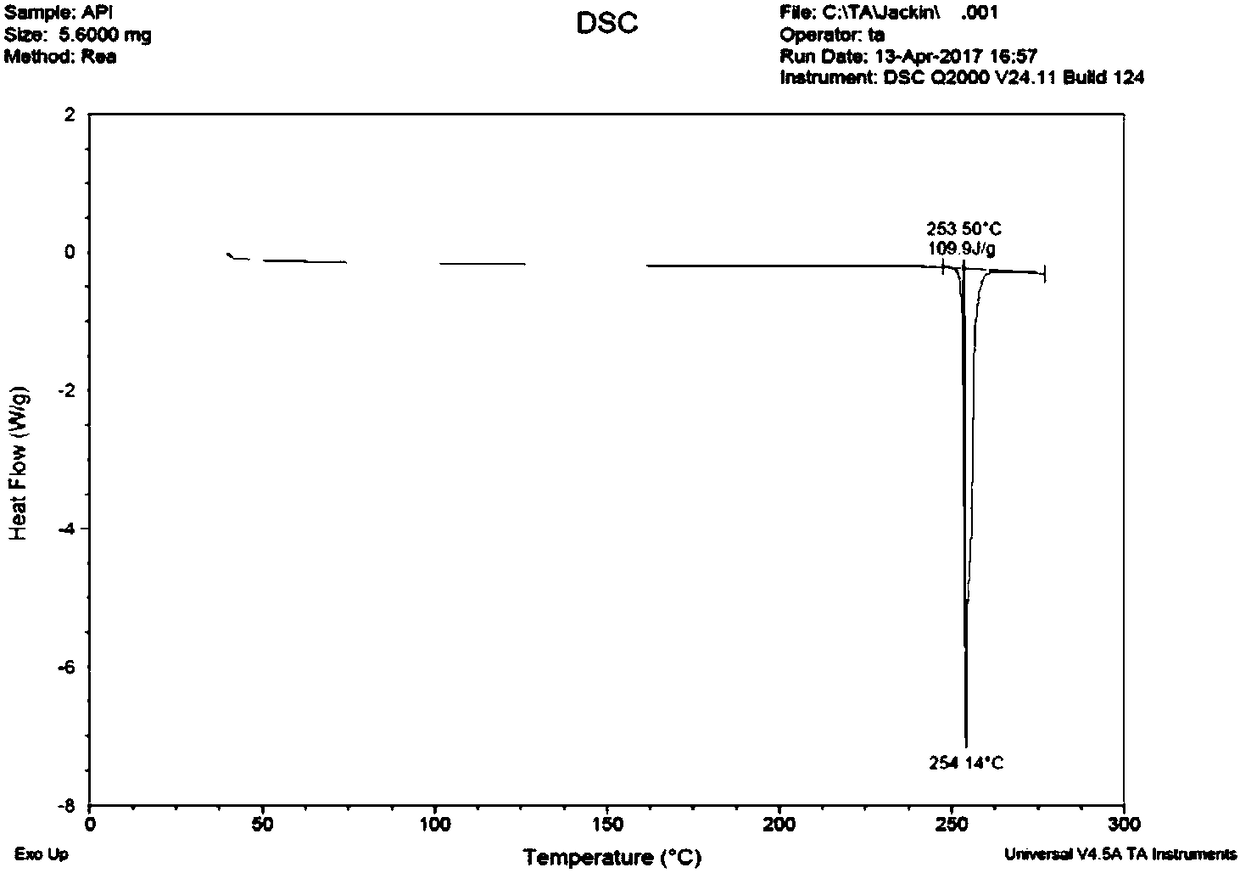
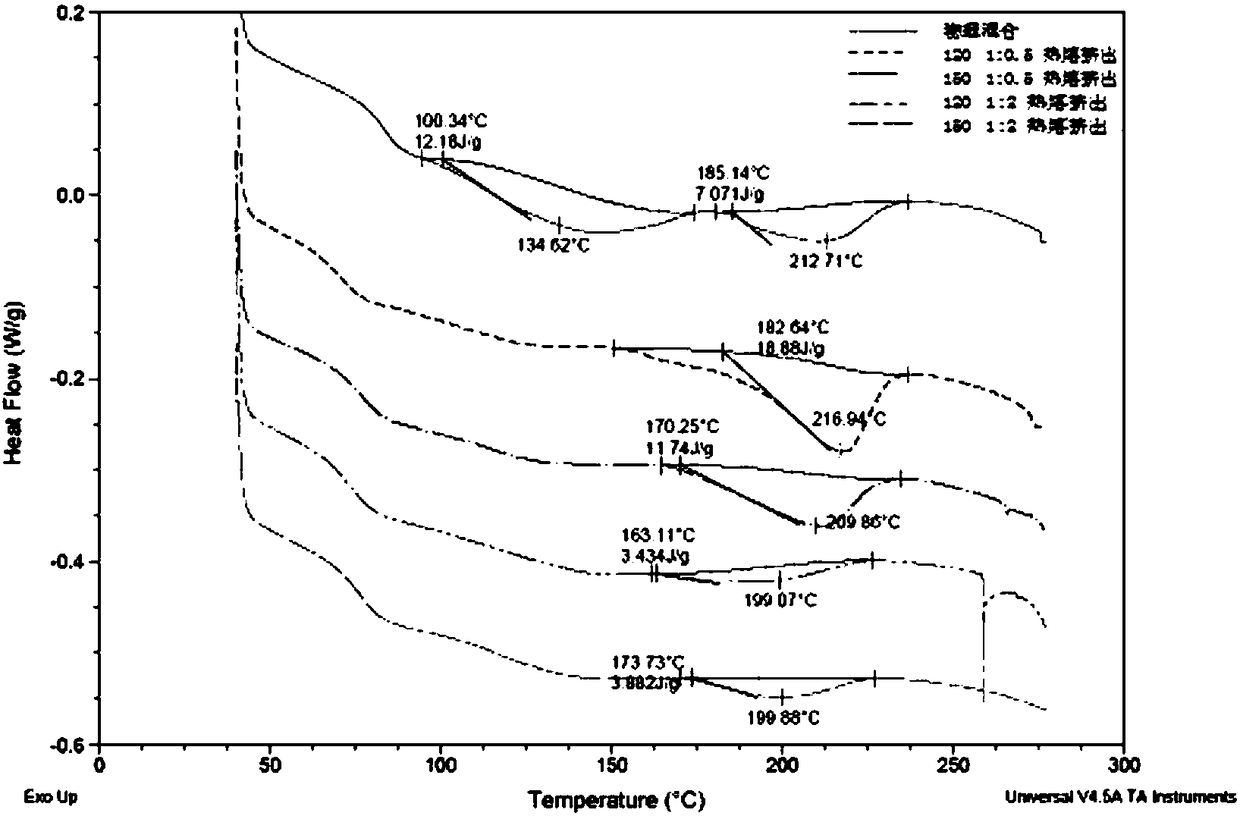
Aprepitant medicine composition and method for improving bioavailability of Aprepitant medicine composition
A technology for aprepitant and composition, which is applied in the field of aprepitant pharmaceutical composition and its preparation, can solve the problems of reduced solubility and dissolution rate, easy recrystallization, large amount of auxiliary materials, etc., and achieves improved solubility and reduced Extrusion temperature, effect of maintaining stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The selection of embodiment 1 carrier
[0037] The present invention screens the commonly used carrier types, and finally selects the enteric carrier material as the main carrier of aprepitant drug, selected from cellulose acetate phthalate, cellulose acetate trimellitate, cellulose acetate succinate, ortho Methylcellulose phthalate, Ethylhydroxymethylcellulose phthalate, Hydroxypropylmethylcellulose phthalate, Hydroxypropylmethylcellulose acetate succinate, Hydroxypropyl acetate maleate methyl cellulose, hydroxypropyl methyl cellulose trimellitate, carboxymethyl ethyl cellulose, polyvinyl butyrate phthalate, polyvinyl acetate phthalate, methyl One or more of acrylic acid / ethyl acrylate copolymer and methacrylic acid / methyl methacrylate copolymer. And for further screening, the enteric carrier material is preferably selected from hydroxypropylmethylcellulose acetate succinate (HPMCAS).
[0038] HPMCAS is a mixed ester of acetic acid and succinic acid of HPMC. It is a ...
Embodiment 2
[0043] The selection of embodiment 2 nonionic surfactants
[0044] Span is a kind of non-ionic surfactant, which is formed by esterification of sorbitol and various long-chain fatty acids (lauric acid, palmitic acid, stearic acid, oleic acid, etc.), and is divided into mono-, di-, and tri-esters. The trade name is Span. The types include Span20, Span40, Span60, Span65, Span80, Span85, and the molecular structure of Span contains hydrophilic sorbitol and hydrophobic fatty acids, and the HLB value is usually in the range of 2-9. It is insoluble in water and not easy to disperse in water, but soluble in ethanol, ether and other organic solvents. It belongs to oil-soluble non-ionic non-ionic surfactant and is a water-in-oil (W / O) emulsifier.
[0045] The Span variety can be used as a solubilizer in the pharmaceutical industry. The present invention screens nonionic surfactants one by one, and finally selects Span as the solubilizer for the auxiliary enteric carrier. The data in t...
Embodiment 3
[0046] Embodiment 3 preparation of aprepitant pharmaceutical composition
[0047] 1. Prescription: The specific composition and dosage of the aprepitant pharmaceutical composition are shown in Table 2:
[0048] The prescription and dosage of table 2 aprepitant pharmaceutical composition, the unit dose of aprepitant is calculated by 80mg
[0049]
[0050] 2. Preparation method:
[0051] (1) Aprepitant, enteric carrier material, and nonionic surfactant are pulverized respectively according to the consumption in Table 1, cross a 40-60 mesh sieve, add in a mixer and mix uniformly; or directly mix the above materials according to Table 1 The amount is put into the twin-screw extruder;
[0052] (2) According to the melting point or glass transition temperature of the drug and excipients, set the extrusion temperature of different sections of the hot-melt extruder to 120-150°C, specifically Zone2 120°C, Zone3 120°C, Zone4 120°C, and Zone5 150°C , Zone6 150°C, Zone7 150°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com